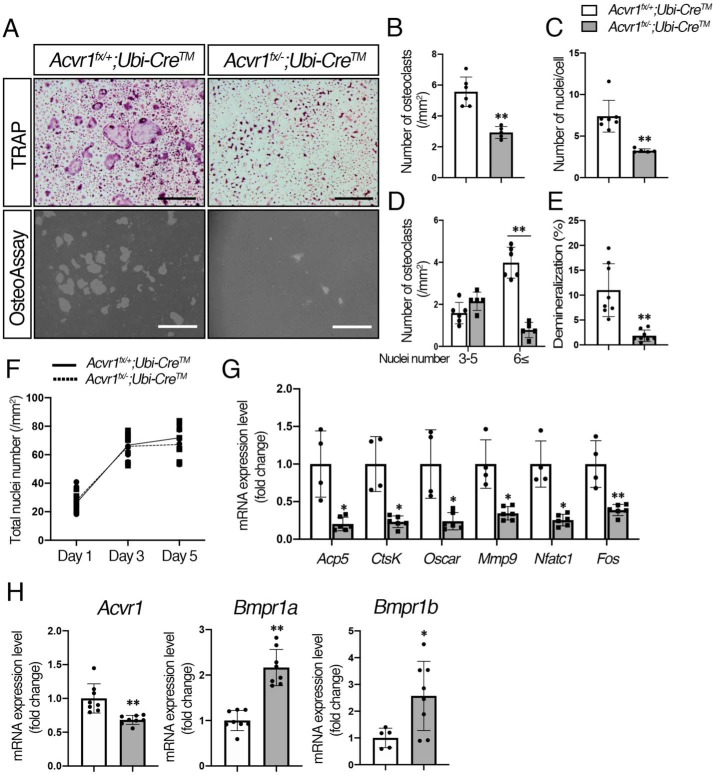
Figure 3.
BMP signaling via ACVR1 promoted RANKL-dependent osteoclastogenesis. A, for TRAP staining, BMMs from control (Acvr1fx/+;Ubi-CreTM) and Acvr1 cKO (Acvr1fx/−;Ubi-CreTM) mice were seeded in 48-well plates (1.5 × 104 cells/well) with M-CSF (20 ng/ml) and RANKL (50 ng/ml) for 5 days, and Cre activity was induced by 4-OHT (100 ng/ml) at the same time. For the demineralization assay, BMMs were seeded onto OsteoAssay Surface plates (1.0 × 104 cells/well) and incubated for 7 days. Scale bars, 500 μm. B, TRAP-positive cells containing three or more nuclei were counted as osteoclasts. n = 6. C, number of nuclei per cell was analyzed. n = 6. D, relative number of small osteoclasts (i.e. those with three to five nuclei) and large osteoclasts (i.e. those with six or more nuclei) were quantified. n = 6. E, demineralized pits generated by osteoclasts was visualized by von Kossa staining. The demineralization area was measured using ImageJ. n = 8. F, after staining nuclei with DAPI, the total number of nuclei in culture was quantified during RANKL treatment from day 1 to day 5. n = 6. G, after culturing for 5 days, total RNA was extracted from osteoclasts, and the expression levels of osteoclast marker genes were assessed by qRT-PCR. n = 4 (control), 6 (cKO). H, comparison of expression levels of Acvr1, Bmpr1a, and Bmpr1b between controls, and Acvr1 cKO osteoclasts were measured by qRT-PCR. n = 8. Values represent the mean ± S.D. Differences were assessed by Student's t test, *, p < 0.05; **, p < 0.01.