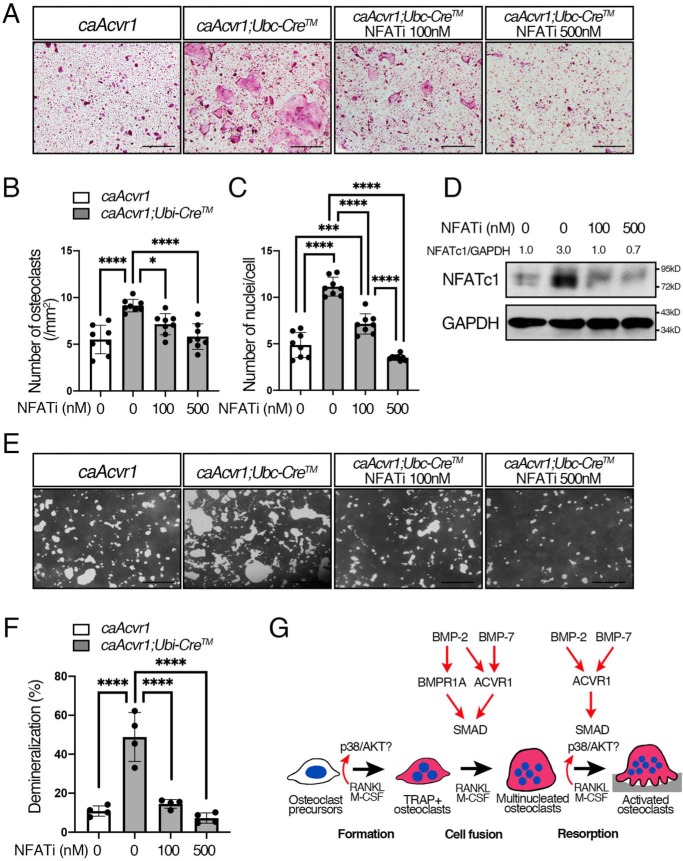
Figure 8.
Inhibition of calcineurin-mediated NFATc1 activation decreased osteoclast differentiation and activity of caAcvr1-mutant cells. A, BMMs from control (caAcvr1) and caAcvr1-mutant (caAcvr1;Ubi-CreTM) mice were incubated with M-CSF (20 ng/ml), RANKL (100 ng/ml), and 4-OHT(100 ng/ml) for 5 days. Cells were treated with an NFAT inhibitor (VIVIT peptide) every other day, and TRAP staining was conducted. Scale bars, 500 μm. B, TRAP-positive cells containing three or more nuclei were counted as osteoclasts. The number of nuclei per cell was analyzed. n = 8. C, BMMs from control (caAcvr1) and caAcvr1-mutant (caAcvr1;Ubi-CreTM) mice were incubated with the NFAT inhibitor for 2 days, and the protein lysate was harvested. The protein levels of NFATc1 were normalized to GAPDH using ImageJ. Fold increases of protein levels are relative to untreated control cells. Representative images of protein bands are shown. n = 3. D, cells were incubated with M-CSF (20 ng/ml), RANKL (100 ng/ml), and 4-hydroxytamoxifen (100 ng/ml) for 7 days. The demineralized pits generated by osteoclasts were visualized by von Kossa staining. Scale bars, 500 μm. E, demineralized area was measured using ImageJ. n = 4. Values represent the mean ± S.D. Differences were assessed by one-way ANOVA, followed by a Tukey test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. F, schematic diagram showing how BMP signaling through each type I receptor differentially regulates RANKL-induced osteoclastogenesis.