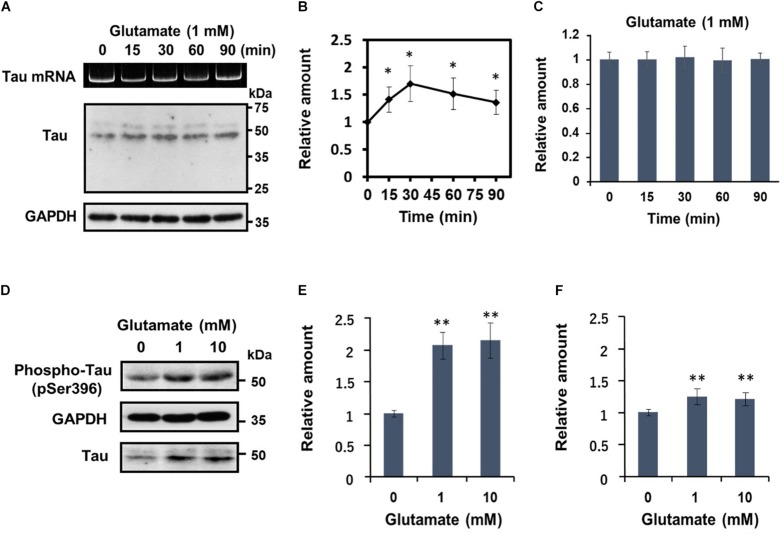
FIGURE 3.
Glutamate-responsive translational activation of tau mRNA is transient, but induces AD-relevant phosphorylation of the newly synthesized tau protein. (A) Time course analysis of the glutamate-dependent increase of tau protein levels. Differentiated SH-SY5Y cells were treated with 1 mM glutamate for the time indicated and tau protein from each cell extract was analyzed by Western blotting. GAPDH served as a loading control. RT-PCR analysis of tau mRNA is also shown. (B) The signal intensity of tau protein at each indicated time point was expressed relative to that of GAPDH. Each value represents the mean and standard error obtained from five independent experiments. ∗P < 0.05 versus control (one-way ANOVA, followed by Tukey–Kramer post hoc test). (C) Quantitative RT-PCR of tau mRNA at each time point is shown. (D) Differentiated SH-SY5Y cells were treated with different concentrations of glutamate for 30 min, and total tau protein and phosphorylated tau (anti-tau pSer396) was detected by Western blotting. The amount of phosphorylated tau protein was expressed relative to that of GAPDH (E) or total tau (F). Each value represents the mean and standard error obtained from four independent experiments. ∗∗P < 0.01 versus control (one-way ANOVA, followed by Tukey–Kramer post hoc test).