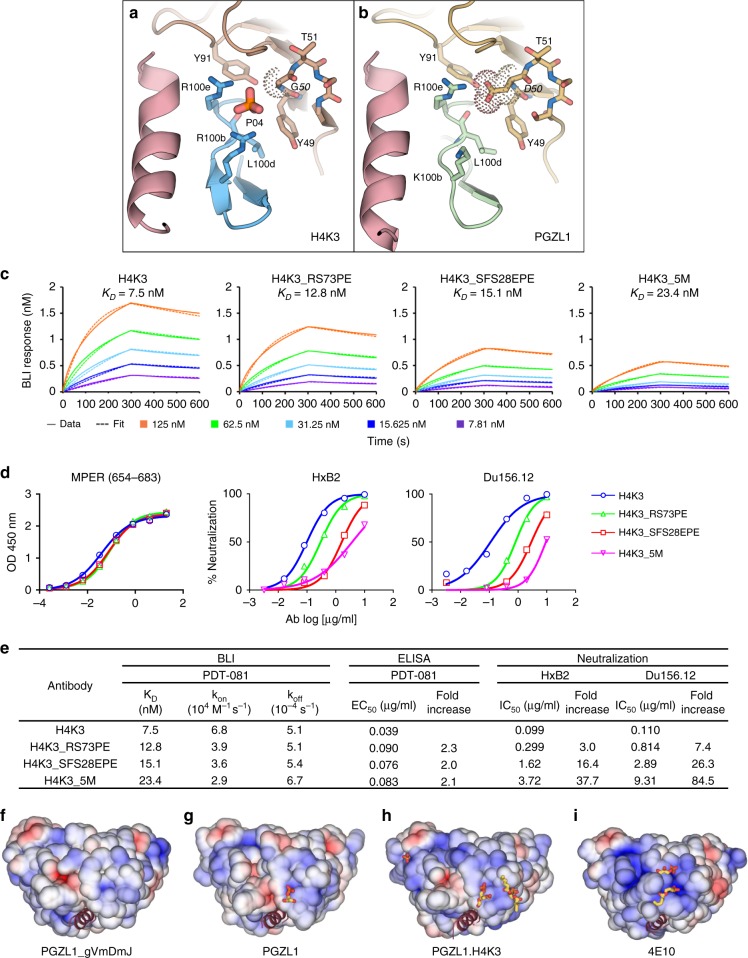
Fig. 6.
MPER-induced PO4-binding site of H4K3 and PGZL1 lipid site mutant characterization and electrostatics. a, b Stick rendering of the PO4-binding site in (a) H4K3 and (b) PGZL1. The side chain at LC position 50 in each antibody is surrounded by dots. Select HC residues of the two antibodies are shown in blue (a) and green (b), and the LC is shown in brown. MPER is shown as a pink ribbon. c Binding of H4K3 lipid-binding site mutants to immobilized MPER peptide by BLI. d Binding of H4K3 lipid-binding site mutants to MPER peptide by ELISA and neutralization (log IC50) of HxB2 and Du156.12. e BLI, ELISA binding to MPER peptide, and neutralization statistics. f–i Surface rendering, along the MPER helical axis (red ribbon), of the solvent accessible electrostatic potential contoured at ± 5 kT/e for (f) PGZL1 gVmDmJ, (g) PGZL1, (h) H4K3, and (i) 4E10. Observed lipid fragments and anions are shown as sticks. Source data for h and i are provided as a Source Data file.