Figure 1.
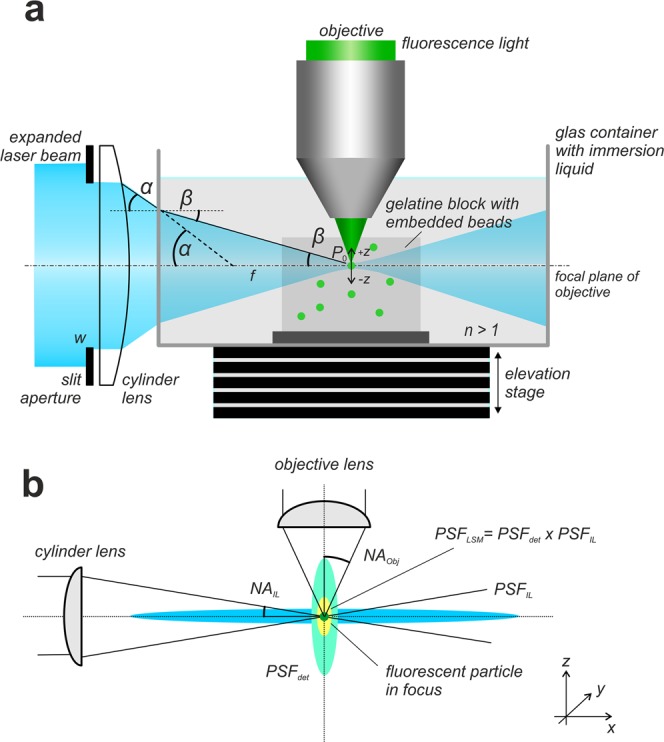
Recording of fluorescent beads. (a) In a light sheet microscope as described in3 the light sheet is generated by a sole cylinder lens and a slit aperture located directly in front of it. Incident on the slit aperture is a Gaussian distributed laser beam that has been expanded to a diameter of about 20 mm by a Galilean beam expander. Specimen and the tip of the objective are immersed in a water filled glass container. A gelatin block with embedded fluorescent particles of 200 nm diameter is placed in the focal region of the light sheet. If the system is correctly adjusted, a small number of fluorescent particles best possibly fits the focal line of the cylinder lens, as well as the focal plane of the objective. For recording, the glass container is stepwise moved vertically through the slight-sheet, while an image is recorded at each position. Since the objective (or the water-proofed protection cap mounted in front of it, respectively) is submerged in the specimen container, all optical path lengths remain constant during the recording procedure. When entering the specimen chamber, the fan angle α of the light sheet changes to β. However, due to the relation NALs = sin(α) = nsin(β) the numerical aperture NALs of the light sheet remains unchanged. (b) A fluorescence emitting sub-resolution particle that is located in the focus of a correctly adjusted light sheet microscope is subjected to an illumination point spread function PSFIL describing the spatial distribution of the excitation light close to the particle and to a detection point spread function PSFdet describing the near field distribution of the emitted fluorescence light collected by an objective of numerical aperture NAObj. The point spread function PSFLSM of the light sheet microscope can be described as the elementwise product PSFIL x PSFdet.
