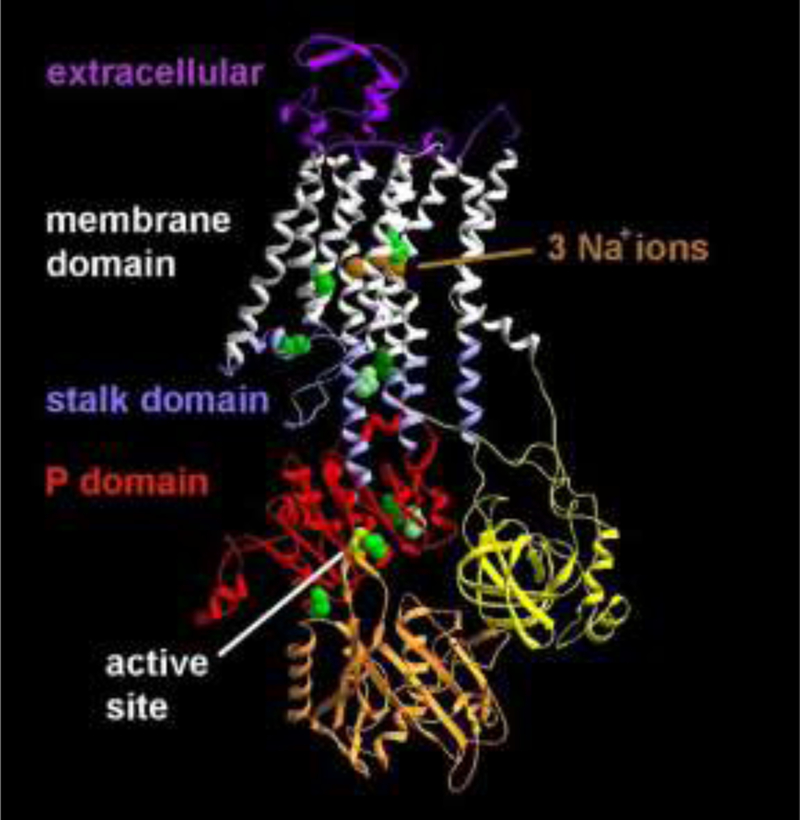
Figure 1: Protein structure of the ATP1A3 Na,K-ATPase.
The sodium-bound form of the ATP1A3 Na,K-ATPase is shown in ribbon view, with the backbone locations of mutated amino acids in green and yellow. Three mutations were in the stalk domain. Two mutations were in the membrane domain not far from the ions, and five were in the P (phosphorylation) domain, including the aspartate (yellow) in the active site that is phosphorylated and dephosphorylated during each cycle of ion transport.