Abstract
Objective—
Hypoxia-induced mitogenic factor (HIMF, also known as FIZZ1 or resistin-like molecule-α) is an etiological factor of pulmonary hypertension (PH) in rodents, but its underlying mechanism is unclear. We investigated the immunomodulatory properties of HIMF signaling in PH pathogenesis.
Approach and Results—
Gene-modified mice that lacked HIMF (knockout) or overexpressed HIMF human homolog resistin (hresistin) were used for in vivo experiments. The pro-PH role of HIMF was verified in HIMF knockout mice exposed to chronic hypoxia or hypoxia/sugen. Mechanistically, HIMF/hResistin activation triggered the high-mobility group box 1 (HMGB1) pathway and receptor for advanced glycation end products (RAGE) in pulmonary endothelial cells (ECs) of hypoxic mouse lungs in vivo, and in human pulmonary microvascular ECs (PMVECs) in vitro. Treatment with conditioned medium from hresistin-stimulated human PMVECs induced an autophagic response, BMPR2 defects, and subsequent apoptosis-resistant proliferation in human pulmonary vascular (PV) smooth muscle cells (SMCs) in an HMGB1-dependent manner. These effects were confirmed in ECs and SMCs isolated from pulmonary arteries of patients with idiopathic PH. HIMF/HMGB1/RAGE-mediated autophagy and BMPR2 impairment were also observed in PVSMCs of hypoxic mice, effects perhaps related to FoxO1 dampening by HIMF. Experiments in EC-specific hresistin-overexpressing transgenic mice confirmed that EC-derived HMGB1 mediated the hresistin-driven pulmonary vascular remodeling and PH.
Conclusion—
In HIMF-induced PH, HMGB1-RAGE signaling is pivotal for mediating EC-SMC crosstalk. The humanized mouse data further support clinical implications for the HIMF/HMGB1 signaling axis and indicate that hresistin and its downstream pathway may constitute targets for the development of novel anti-PH therapeutics in humans.
Keywords: resistin, RELMα, FIZZ1, DAMP, RAGE
Introduction
Pulmonary hypertension (PH) is a lethal and multifactorial disease characterized by plexiform lesions and vascular remodeling. Its pathogenesis remains enigmatic because of its tremendous complexity. We previously reported that a protein of the resistin-like molecule (RELM) family is upregulated in the chronically hypoxic lung PH model.1 This protein, which we named hypoxia-induced mitogenic factor (HIMF, also known as FIZZ1 or RELMα) has potent mitogenic effects. We have reported that in vivo, HIMF knockdown blocks hypoxia-induced pulmonary vascular remodeling and right heart hypertrophy,2 whereas HIMF gene transfer into rat lungs causes PH development, with exacerbated vascular remodeling and PH hemodynamic changes.2 Thus, HIMF appears to cause PH. However, the immunoregulatory mechanisms underlying the etiologic role of HIMF signaling remain poorly characterized.
Data from our previous studies showed that HIMF induces endothelial cell (EC) apoptosis,3 suggesting that HIMF triggers some endogenous danger signals in response to the hypoxia-induced lung tissue injury. As a key damage-associated molecular pattern (DAMP) molecule, high-mobility group box 1 (HMGB1) is released from apoptotic or necrotic cells, and from cells activated by cytokine stimulation.4,5 HMGB1 was recently recognized as a pro-autophagic factor6 that signals predominantly through the receptor for advanced glycation end products (RAGE).7,8 HMGB1 and RAGE have each been implicated in PH pathogenesis;9-11 however, whether the HMGB1/RAGE axis is regulated by HIMF signaling during PH development is still unclear. Recent studies12 have suggested that PH development is associated with increased lung autophagy and downregulation of bone morphogenetic protein receptor 2 (BMPR2). The dysfunction of BMPR2 might be indirectly caused by RAGE activation.10 These findings led to our hypothesis that HIMF activates HMGB1 released from injured ECs to act as a pivotal mediator for proliferation of smooth muscle cells (SMCs). Such a pathway would relate to the aberrations of autophagy and BMPR2 through RAGE signaling and thereby contribute to PH initiation and progression.
We investigated the role of HIMF and its human homolog, resistin (hresistin), in controlling the HMGB1/RAGE pathway and its downstream effectors. We also examined whether this pathway mediates the EC-SMC crosstalk required for pulmonary vascular remodeling. We generated gene-modified mice with HIMF-deficiency or with humanized hresistin overexpression to dissect the immunomodulatory properties of HIMF/HMGB1 signaling. The mechanistic features were further interrogated by in vitro assays with primary human PH-related vascular cells. Our results showed the HIMF/HMGB1 signaling axis to be a critical hub that orchestrates the interface of two key PH elements, EC dysfunction and SMC proliferation. These findings suggest that interventions to disrupt the axis-driven cellular interplay may represent effective anti-PH therapeutic strategies.
Methods
All animals, antibodies, and cell lines used in this study are detailed in the Major Resources Tables in the online-only Data Supplement.
Animals and PH Models
Age-matched 6- to 12-week-old C57BL/6J mice (Charles River Laboratories, Wilmington, MA), and 125-150 g Sprague-Dawley rats (Charles River), were used for all experiments. Given reports that estrogens exhibit protective effects in classical rodent PH models, including the chronic hypoxia and sugen/hypoxia models,13,14 and the finding that the damage-associated inflammatory response is more profound in male rodents with PH than in corresponding female rodents,15 we used only male mice and rats to produce a more severe disease and dissect the HIMF-regulated DAMP signaling. FIZZ1 (RELMα) traditional knockout (KO) mice on C57BL/6J background (≥7 generations) were generated and verified as previously described.16 Animals were maintained on a 12/12-hour light/dark cycle with access to normal laboratory diet (Teklad global 18% protein rodent diet, Envigo, USA) and chlorinated water ad libitum. Cage bedding was also from Envigo (7097 Teklad corncob bedding). Animal housing and experimental protocols were approved by the Animal Care and Use Committee of Johns Hopkins University. For induction of the chronic hypoxia-induced PH model, mice were exposed to 10.0% O2 (hypoxia) for 4 or 28 days and then sacrificed and processed as described previously.17 Control mice were exposed to normal room air (20.8% O2, normoxia). For validation with the sugen-hypoxia (Su/Hx) PH model, mice were exposed to hypoxia and injected subcutaneously with VEGFR2-inhibitor sugen5416 (20 mg/kg, S8442, Sigma-Aldrich; St. Louis, MO) once a week according to published protocol.18 PH was also induced in rats by one subcutaneous injection of 20 mg/kg sugen5416 and exposure to hypoxia according to a published approach.19 On day 0, rats were injected intraperitoneally (i.p.) with 4 mg/kg of our recently generated anti-HIMF therapeutic antibody, which was developed in cooperation with our commercial partners Creative Biolabs (Shirley, NY) and Lonza (London, England),20 or control IgG1 (isotype control provided by Lonza). On day 4 after Su/Hx treatment, rats were killed and processed for histological examination. We ensured that experiments were unbiased by following the recent guidelines for PH preclinical and translational research.21,22 Animals were randomized to each group, and group sizes were determined by power calculations. Investigators who assessed the imaging, hemodynamics, and histological outcomes in all PH animal models were blinded to group assignment.
Generation of Transgenic Mice That Overexpress hResistin
We developed tetracycline-switchable hresistin knock-in transgenic mice that allow for spatially and temporally controlled overexpression of recombinant hresistin in lung ECs (“humanized mice”). This tool was generated with two transgenes: one is the tetracycline trans-activator (tTA), which is driven by EC-specific promoter angiopoietin receptor (Tie-2), and the second contains human resistin cDNA followed by a FLAG tag. hResistin transgene-positive founders were identified and crossbred with Tie2-tTA mice to obtain the double transgenic mice. Mothers were treated with doxycycline throughout pregnancy and during the weaning period to block expression of hresistin. Doxycycline was withdrawn from mothers approximately 1 month after parturition. hResistin was overexpressed for 4 days before bronchoalveolar lavage fluid (BALF) and lung tissues were collected for study of the early inflammation stage, or for 6 weeks before terminal right heart catheterization.
Hemodynamic Analysis and Fulton Index Measurement
Right ventricular function was assessed in vivo by a pressure-volume catheter as described previously17 in wild-type (WT) and HIMF KO mice on post-hypoxic day 28 and in humanized mice at 6 weeks post-switch-on. The mice were anesthetized with 75 mg/kg i.p. urethane, 5 mg/kg i.p. etomidate, and 1 mg/kg i.p. morphine. Then they underwent tracheostomy and were ventilated with 6-7 μL/g tidal volume at 130 breaths/minute. Right ventricular systolic pressure (RVSP) was measured and data were collected and analyzed with the AD Instruments Powerlab 8/35 (AD Instruments, Colorado Springs, CO) and Millar MPVS Ultra (Millar Instruments, Houston, TX). Right heart hypertrophy induced by Hx/Su (4 weeks), hypoxia (6 weeks), or hresistin humanized overexpression (6 weeks) was also assessed. The heart was dissected free of all major vessels, separated into right ventricle (RV) and left ventricle (LV) plus septum (S), and weighed. The Fulton index (RV/LV+S) was determined as described previously.17
Pulmonary Vascular Remodeling Analysis
We determined pulmonary vascular remodeling of the hypoxic and humanized mice as previously published.17 Lung sections were dual labeled with antibodies to von Willebrand factor (vWF; A008202, Dako, Glostrup, Denmark) and α-smooth muscle actin (α-SMA; M085129, Dako), which stain endothelium and vascular smooth muscle with HRP-DAB (brown, Vector Labs, SK-41001) and AP (red, SK-51001, Vector Labs) systems, respectively. An investigator blinded to treatment group assessed remodeling of the lung arteries and arterioles. The examination procedure and criteria have been described previously.17
In Vivo Treatment
We introduced pharmacologic inhibitors of the HIMF/HMGB1/RAGE signaling axis into our mouse models. The hypoxic WT mice and Tie-2-hresistin-overexpressing humanized mice were injected i.p. daily with HMGB1 inhibitor ethyl pyruvate (EP, E47808, Sigma, 50 mg/kg),23 RAGE antagonist FPS-ZM1 (553030, EMD Millipore, 1 mg/kg),24 or the cell-permeable autophagic sequestration blocker 3-methyladenine (3-MA, 189490, EMD Millipore, 15 mg/kg).25 Experimental mice were administered these inhibitors daily from post-hypoxia (or post-switch-on in the transgenic mice) day 0 to day 4. On day 4, the left lung was fixed in 10% formalin for histologic study, and other lung pieces were snap frozen and stored at −80°C for use in immunoblot analysis.
Human Cell Culture and In Vitro Treatment
Human pulmonary microvascular endothelial cells (PMVECs, CC-2527, Lonza, Walkersville, MD) and human pulmonary artery (vascular) smooth muscle cells (PVSMCs, CC-2581, Lonza) were used in this study within passages 5-9. For conditioned medium preparation, PMVECs were serum- and growth factor-starved for 16 hours and then treated with 100 ng/mL lab-made recombinant hresistin or vehicle. Preliminary studies were performed to optimize both incubation time and recombinant protein concentrations (data not shown). After 16 hours, the medium was collected and tested by ELISA or applied to starved hPVSMCs. In parallel experiments, conditioned medium from hresistin-treated ECs was applied to PVSMCs pretreated with HMGB1 antagonists Box-A (REHM012, Tecan, 2 μg/mL) or EP (5 μM), RAGE inhibitor FPS-ZM1 (553030, EMD Millipore, 200 nM), or bafilomycin A1 (BA1, 196000, Sigma, 100 nM), which inhibits autophagosome/autophagolysosome formation.26 These conditioned medium-treated PVSMCs were collected at 16 hours post-stimulation for western blot analysis. In some experiments, cells seeded on the fibronectin-coated coverslips (12 mm round, Neuvitro, GG-12-fibronectin) were treated as described above and then used for immunocytochemistry analysis. To confirm our results, we also used passage-5-9 ECs and SMCs derived from pulmonary type II arteries (1 to 5 mm diameter) of individuals without (donor, control, n = 3) or with idiopathic pulmonary hypertension (IPAH, n = 3). The pulmonary vascular ECs were stimulated by hresistin protein, and the conditioned medium was collected and applied to the isolated SMCs with or without pretreatment by HMGB1/RAGE inhibitors.
Statistical Analysis
Data are presented as the mean ± SEM. At least three independent experiments were performed for in vitro studies, and in vivo animal experiments were repeated independently at least twice. The Shapiro-Wilk test was used to determine the normality of data and the F test was used to assess equality of variances. All data passed normality and equal variance tests. Comparisons between two groups were analyzed by Student’s t test, and comparisons of multiple groups were analyzed by one-way ANOVA followed by the Newman-Keuls post-hoc test. All statistical analyses were performed with Prism 7.0e (GraphPad Software, La Jolla, CA). A p < 0.05 was considered statistically significant.
Extended Materials and Methods for hresistin production, immunohistochemistry, western blot analysis, flow cytometry-based assay, ELISA, and BALF collection are provided in the online-only Data Supplement.
Results
HIMF deficiency ameliorates pulmonary vascular remodeling and PH development
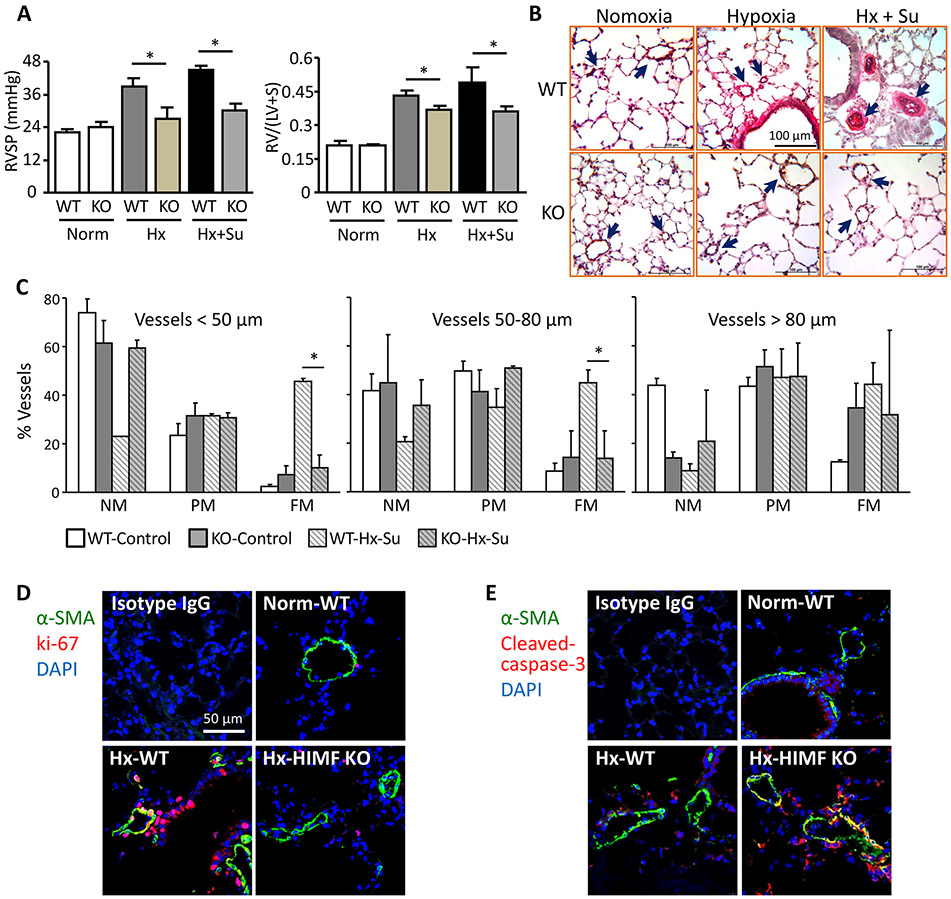
Previous in vivo knockdown of HIMF by short hairpin RNA has suggested that HIMF induces PH in the chronic hypoxia model.2 HIMF silencing partially improved the hemodynamics and pulmonary vascular remodeling.2 In the present study we used HIMF/FIZZ1 KO mice16 to completely abolish this signaling in hypoxic animals. As expected, HIMF genetic ablation prevented the hypoxia-induced increases in RVSP (Figure 1A, left panel), right heart hypertrophy (Figure 1A, right panel), and pulmonary vascular resistance (Figure 1B) seen in WT mice. The inhibition of PH development by HIMF deficiency was further validated by hemodynamic data and vascular remodeling in the PH mouse model induced by hypoxia plus sugen5416 (Figure 1A-1C). Histologic analysis showed that hypoxic HIMF KO mice had less arterial muscularization and small pulmonary vessel thickening throughout the lung vascular bed during late PH development stage than did hypoxic WT mice (Figure 1B). Mechanistically, immunofluorescence staining of lung tissues for ki-67 and cleaved caspase-3 revealed impaired proliferation of the α-SMA-positive PVSMCs in HIMF-deficient hypoxic lungs during the early inflammatory phase (Figure 1D and 1E), indicating that loss of the HIMF pathway mitigates the PH phenotype of PVSMCs.
Figure 1.
Pulmonary vascular remodeling and pulmonary hypertension development in HIMF-deficient mice. A, Hemodynamic analysis (left) and Fulton index (right). Right ventricular systolic pressure (RVSP) was measured. Wild-type (WT) mice subjected to hypoxia (Hx) with or without sugen (Su) 5416 exhibited increased RVSP, but the enhancement of RVSP was significantly lower in HIMF knockout (KO) mice. The mouse hearts were bisected and the RV/LV+S was also determined. HIMF depletion decreased right heart hypertrophy induced by hypoxia or by Hx/Su in mice. Data represent means ± SEM (n ≥ 6). *p<0.05. B, HIMF gene deficiency prevented pulmonary vascular remodeling in mouse hypoxic lungs. Microphotographs from lung tissue sections stained for von Willebrand factor (vWF, brown) and α-smooth muscle actin (α-SMA, red) are shown to define non-muscularized (NM), partially muscularized (PM), and fully muscularized (FM) intra-alveolar small vessels. Arrows mark muscularized small vessels. C, Percent muscularization of small pulmonary vessels in mouse hypoxic lung. HIMF gene deletion caused resistance to vascular remodeling. Data represent means ± SEM (n = 6). *p<0.05. D and E, Co-localization analysis of hypoxic lung tissues from WT and HIMF-KO mice. Sections of lung tissue after 4 days of hypoxia were stained with anti-Ki-67 (D, red, proliferation marker) or anti-cleaved caspase-3 (E, red, apoptosis marker), co-stained with anti-α-SMA (green), and counter-stained with DAPI (blue). Representative images from 4 individual lung samples per group. Magnification: 400X.
HIMF triggers HMGB1/RAGE signaling in pulmonary ECs
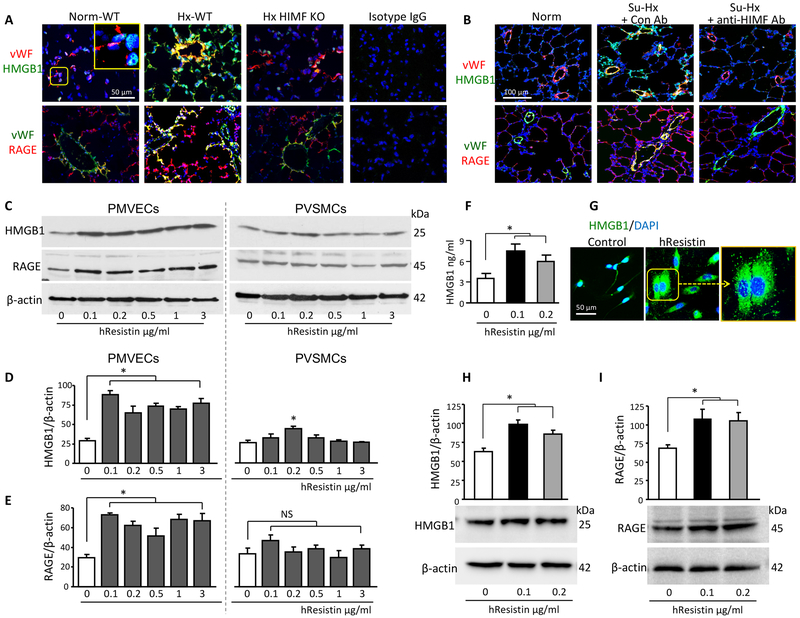
Next we explored the endogenous mechanisms of HIMF immunoregulation in the classic hypoxia-induced mouse PH model. We found that the key DAMP players, HMGB1 and RAGE, were highly induced in the hypoxic lungs and that both colocalized with vWF+ PMVECs during the early inflammatory stage (Figure 2A). However, in KO mice, loss of HIMF attenuated expression of the HMGB1/RAGE axis in hypoxia-induced inflammatory lung tissues (Figure 2A). Similar HIMF-dependent HMGB1/RAGE activation was also observed in pulmonary artery ECs of the Su/Hx-treated rats (Figure 2B). Consistent with this finding, our in vitro study showed that stimulation with hresistin, the human homolog of HIMF, significantly upregulated the protein levels of HMGB1 and RAGE in primary human PMVECs, as detected by western blotting (Figure 2C-2E). In human PVSMCs, hresistin treatment also enhanced the HMGB1 protein expression, but the magnitude was markedly lower than that in the hresistin-stimulated ECs (Figure 2C-2E). In contrast, hresistin failed to alter RAGE production in the PVSMCs (Figure 2C-2E). ELISA further revealed elevated HMGB1 levels in the cell supernatant of hresistin-stimulated human PMVECs (Figure 2F). Using immunocytochemical microscopy, we observed stronger HMGB1 signal and enhanced release of this DAMP in hresistin-treated PMVECs (Figure 2G). Intriguingly, the ECs isolated from small pulmonary arteries of patients with IPAH exhibited heightened basal production of both HMGB1 and RAGE proteins (Figure 2H and 2I), and hresistin significantly enhanced the expression of these DAMP proteins (Figure 2H and 2I). The expression patterns of HMGB1 and RAGE suggested that they have a role in mediating the interplay between PH-related vascular cells.
Figure 2.
HIMF/hResistin induces HMGB1/RAGE expression and HMGB1 release in pulmonary vascular endothelial cells. A, Double-color immunofluorescence analysis of lung tissue after 4 days of hypoxia. Sections were stained with anti-HMGB1 or anti-RAGE and co-stained with endothelial cell marker anti-von Willebrand factor (vWF). The inset, at higher magnification, depicts the HMGB1 nuclear localization in the normoxic wild-type (WT) mouse lungs. Original magnification: 400X. Images are representative of 4 individual lung samples. Pulmonary microvascular endothelial cells (PMVECs) from hypoxic WT lungs exhibited strong signals for HMGB1 and RAGE. In contrast, signal intensities were low in PMVECs from hypoxic HIMF knockout (KO) lungs. B, Rats were injected i.p. with 4 mg/kg anti-HIMF or control (IgG1) antibody and then exposed to Su-Hx treatment. After 4 days, rat lung tissues were analyzed by immunofluorescence for HMGB1 and RAGE expression. Representative images from 6 individual lung samples per group are shown. C, Western blotting of hresistin-treated human (h) PMVECs (left panel) or PVSMCs (right panel). D and E, Quantitative analysis of data in C. The ratio of HMGB1 (D) or RAGE (E) to β-actin was analyzed. Data represent means ± SEM (n = 6). *p<0.05. F, Protein level of HMGB1 in supernatants from human PMVECs was assayed by ELISA after 18 hours of hresistin stimulation. Values represent mean ± SEM of duplicate determinations. Representative results of 3 independent experiments. *p<0.05 vs vehicle-treated controls. G, hPMVECs treated with hresistin (100 ng/mL) were stained with anti-HMGB1 antibody (green) and DAPI (blue). Original magnification: 400X. Boxed area of hresistin-treated cells is enlarged in the right panel to show HMGB1 cytoplasmic accumulation and release. Representative images are from 6 individual samples per group. H and I, Western blot analysis of HMGB1 (H) and RAGE (I) expression in hresistin-treated pulmonary artery ECs isolated from IPAH patients. The results shown are representative of n = 3 IPAH-ECs. Experiments were repeated three times. Data represent means ± SEM (n = 3, pooled data of three repeat experiments). *p<0.05.
HIMF/HMGB1 signaling axis induces EC-SMC crosstalk
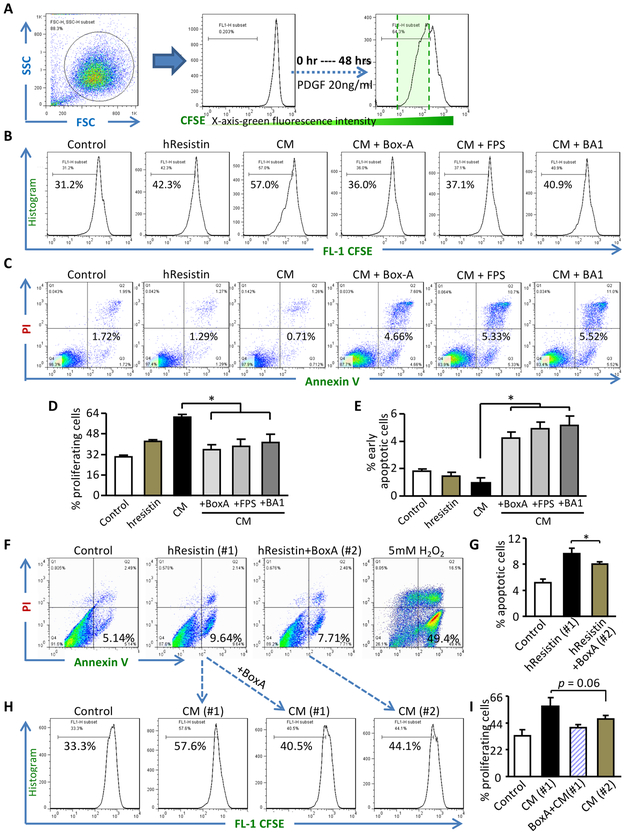
We next investigated whether and how the HIMF-mediated HMGB1/RAGE signaling axis contributes to vascular remodeling. Recombinant Box-A protein, a truncated N-terminal fragment of full-length HMGB1, is a specific HMGB1 antagonist that interacts with RAGE without functional stimulation,5 whereas EP can attenuate the extracellular release of HMGB1 and downregulate the HMGB1-RAGE axis.23 FPS-ZM1 is a specific RAGE antagonistic peptide.24 We used these three HMGB1/RAGE inhibitors to study the HIMF/HMGB1/RAGE axis in vitro and in animal models. Flow cytometry analysis with 5,6-carboxyfluorescein diacetate succinimidyl ester labeling and tracing (Figure 3A) showed that conditioned medium from the hresistin-treated human PMVECs promoted the proliferation of human PVSMCs more potently than did direct stimulation with hresistin alone (Figure 3B and 3D). These data strengthened our previous finding that HIMF promotes EC-mediated alteration of PVSMCs to a proliferative phenotype.3 The hresistin-treated pulmonary artery ECs from IPAH patients also consistently exhibited potentiated PVSMC proliferation (Supplemental Figure ID-IE). Intriguingly, pre-incubation with HMGB1 antagonist or RAGE inhibitor almost completely blocked the enhanced growth of PVSMCs induced by the conditioned medium (Figure 3B and 3D). Concordantly, medium from hresistin-stimulated ECs also inhibited the apoptotic response of starved human PVSMCs. This inhibition was reversed by the addition of HMGB1/RAGE axis blockers (Figure 3C and 3E). HMGB1 suppression mildly attenuated the hresistin-mediated EC dysfunction (Figure 3F and 3G), but conditioned media from ECs treated with HMGB1 inhibitors failed to augment PVSMC proliferation (Figure 3H and 3I). These in vitro data reveal a HIMF-mediated EC-SMC interaction that is dependent on the HMGB1/RAGE axis and suggest that the cytoprotective effects of DAMP are a crucial mechanism that underlies HIMF-driven vascular inflammation and subsequent remodeling.
Figure 3.
HIMF/HMGB1 signaling axis induces apoptosis-resistant proliferation in human pulmonary vascular smooth muscle cells (PVSMCs). A and B, 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling and flow cytometry analysis were used to determine proliferation of human (h) PVSMCs treated with hresistin alone or with conditioned medium (CM) from human pulmonary microvascular endothelial cells (hPMVECs) stimulated with hresistin (100 ng/mL; pretreated 1 hour with or without HMGB1/RAGE/autophagy inhibitors). Platelet-derived growth factor (PDGF, 20 ng/mL) treatment served as a positive control. C, Apoptosis of the CM-treated hPVSMCs was assessed with the Annexin V-FITC kit. D and E, Quantitative analysis of data in B and C, respectively. Data represent means ± SEM (n = 4-5). *p<0.05. F and G, As detected by Annexin V-FITC assay, hresistin (100 ng/mL, 16 hours) promoted apoptosis of hPMVECs (#1). Pretreatment with HMGB1 antagonist Box-A fragment moderately attenuated the hresistin-induced apoptosis (#2). Hydrogen peroxide (H2O2, 5 mM) treatment served as a positive control. H and I, CFSE assay showed that treatment with CM from #1 EC culture system promoted hPVSMC proliferation. Pretreatment of hPVSMCs with Box-A prevented #1-CM-enhanced cell growth. However, CM from the #2 culture system failed to further augment hPVSMC proliferation compared to that in hPVSMCs incubated with #1-CM. Data represent means ± SEM (n = 4). *p<0.05.
HIMF/HMGB1 downstream elements in the EC-SMC interaction
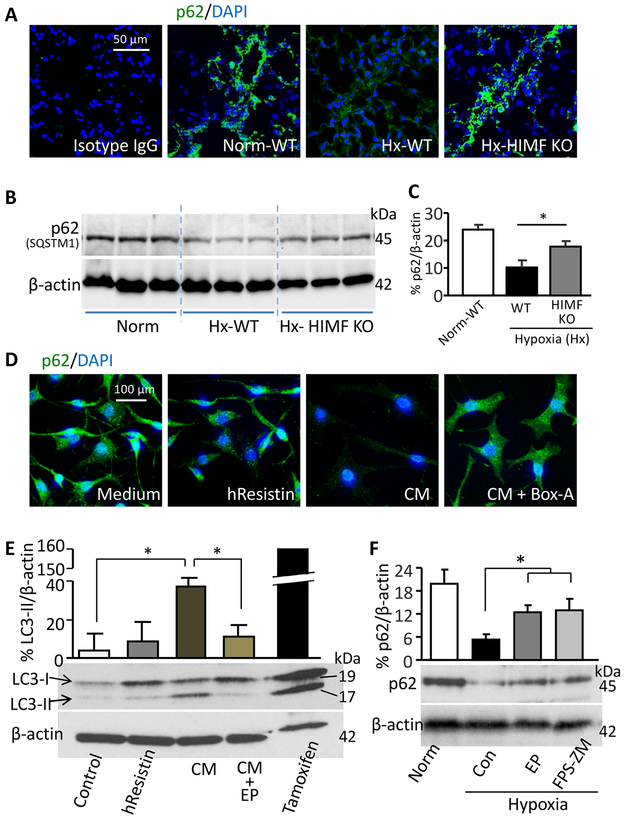
We further explored the downstream effectors responsible for the HIMF/HMGB1-mediated EC-SMC interaction and PVSMC proliferation. In mouse lungs, hypoxia reduced the expression of anti-autophagy marker p62 in the PVSMC-like cells (Figure 4A-4C), but this effect was absent in mice with HIMF gene deletion (Figure 4A-4C), implying the presence of a hypoxia-induced HIMF-mediated autophagic response in lungs. In vitro, direct hresistin treatment had only moderate effects on human PVSMC autophagy, whereas conditioned medium from hresistin-stimulated PMVECs highly upregulated the autophagy marker LC3B-II (Figure 4E) and downregulated p62 (Figure 4D), in support of the in vivo observations (Figure 4A-4C). Pretreatment with HMGB1/RAGE inhibitor abolished the pro-autophagy effects of HIMF-treated ECs on human PVSMCs (Figure 4D and 4E), and the autophagy inhibitor BA1 alleviated the HIMF-driven apoptosis-resistant proliferation of PVSMCs (Figure 3B-3E). We obtained similar results when we used pulmonary artery ECs and PVSMCs derived from IPAH patients (Supplemental Figure IA, IC, IF, and IH). Pharmacologic suppression of HMGB1/RAGE mitigated autophagy in hypoxic mouse lungs (Figure 4F). Both cell culture and animal data suggested that the HIMF/hresistin-induced PVSMC autophagic response is dependent on EC-derived HMGB1-RAGE signaling, which further regulates the pro-PH phenotype of PVSMCs and mediates vascular inflammation in hypoxic lungs.
Figure 4.
HMGB1 signaling mediates HIMF-induced autophagy. A, Sections of mouse lungs on day 4 of hypoxia (Hx) were stained with antibodies to label anti-autophagic marker-p62 (green) and DAPI (blue). Photographs are representative of 4 individual lung samples. Magnification: 400X. B, Western blotting of SQSTM1/p62 expression levels in lung lysates collected from mice on day 4 of hypoxia. C, Quantitative analysis of data in B. Data represent means ± SEM (n = 6 per group). *p<0.05. D, Immunocytochemical analysis of p62 staining for human pulmonary vascular smooth muscle cells (PVSMCs) treated with control (vehicle) medium, with 100 ng/mL hresistin alone, or with conditioned medium (CM) from hresistin-stimulated human pulmonary microvascular endothelial cells (pretreated 1 hour with or without 2 μg/mL Box-A fragment, the specific HMGB1 antagonist). Representative images from 4 individual samples per group are shown. Magnification: 200X. E, Western blotting of CM-treated human PVSMCs that were pretreated with or without HMGB1 inhibitor ethyl pyruvate (EP, 5 μM). Tamoxifen served as a positive control for autophagy. Data represent means ± SEM (n = 6) showing quantitative analysis of LC3-II. *p<0.05. F, Western blot analysis of anti-autophagy marker p62 expression in lung lysates from mice that underwent 4 days of hypoxia and received once-daily i.p. injection of HMGB1 inhibitor EP (50 mg/kg) or RAGE inhibitor FPS-ZM1 (1 mg/kg). The ratio of p62 to β-actin was quantitatively analyzed. Data represent means ± SEM (n = 4–6 animals per group). *p<0.05. KO, knockout; WT, wild-type.
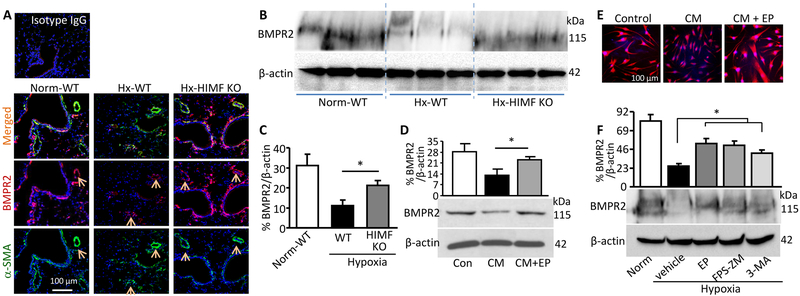
The anti-proliferative factor BMPR2 is another PH causal factor27 involved in RAGE signaling.10 Our hypoxic animal study revealed that HIMF deficiency rescued the impaired expression of BMPR2 in mouse lungs (Figure 5A-5C). This salvaged BMPR2 localized mainly in PVSMCs (Figure 5A), indicating the involvement of HIMF and DAMPs in modulating BMPR2. Therefore, we hypothesized that HMGB1 released from hresistin-stimulated ECs induces BMPR2 dysfunction in PVSMCs and that this dysfunction also contributes to the HIMF-driven PVSMC proliferation and vascular remodeling. Conditioned medium from hresistin-treated primary human pulmonary artery ECs isolated from both control and IPAH patients significantly dampened BMPR2 expression in PVSMCs; however, expression was preserved when PVSMCs (from IPAH patients and controls) were pretreated with HMGB1/RAGE inhibitor (Figure 5D and 5E; Supplemental Figure IA, IB, IF, and IG). Moreover, in vivo application of HMGB1/RAGE blockers reversed hypoxia-induced BMPR2 suppression in mouse lungs (Figure 5F). Thus through EC-SMC interplay, HIMF/HMGB1 signaling downregulates BMPR2 to synergistically potentiate PVSMC growth.
Figure 5.
HIMF/HMGB1 axis suppresses BMPR2 expression. A, Immunofluorescence staining of lung sections after 4 days of hypoxia (Hx). BMPR2 signal was stronger in lungs from HIMF-knockout (KO) mice than in lungs from wild-type (WT) mice. In all three groups, BMPR2 was enriched in perivascular areas. Arrows indicate BMPR2 expression (red) in the α-smooth muscle actin (α-SMA)-positive pulmonary vascular smooth muscle cells (PVSMCs, green). Magnification: 200X. Representative photographs of 4 individual samples per group. B, Western blotting of lung lysates collected from mice after 4 days of hypoxia. C, Quantitative analysis of data in B. Data represent means ± SEM (n = 6 per group). *p<0.05. D, Western blot analysis of BMPR2 expression in human PVSMCs treated with conditioned medium (CM) from HIMF-stimulated human pulmonary microvascular endothelial cells with or without HMGB1 antagonist as described in the legend to Figure 4. Data represent means ± SEM (n = 6). *p<0.05 vs CM treatment alone. E, Representative photographs show immunocytochemistry of CM-treated human PVSMCs. Magnification: 200X. n = 4 individual samples per group. F, Western blot analysis of BMPR2 levels in lung lysates collected from mice that were exposed to 4 days of hypoxia and treated with HMGB1/RAGE antagonists (as described in Figure 4F) or with autophagy inhibitor 3-methyladenine (3-MA, 15 mg/kg). Data represent means ± SEM (n = 4-6 per group). *p<0.05.
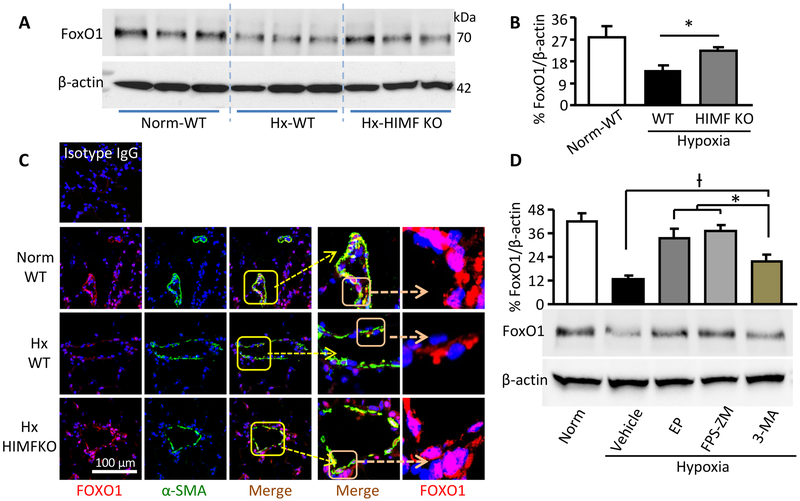
We tried to determine the interaction between the two HIMF downstream events, autophagy and BMPR2, that we had uncovered. HMGB1 and RAGE were associated with other proliferation-related factors, such as signal transducer and activator of transcription (STAT) 310,28 and forkhead box O (FoxO) 1,29 which also may link autophagy with diminished BMPR2.12,30,31 In our mouse hypoxic PH model, blocking HMGB1/RAGE in vivo failed to alter the expression of total STAT3 or Tyr705-phosphorylated-STAT3 (data not shown). In contrast, lung FoxO1 expression was attenuated during early hypoxia (Figure 6A-6C). HIMF gene deletion or HMGB1/RAGE inhibitor administration rescued the hypoxia-impaired FoxO1 (Figure 6A-6D). Histologic analysis of hypoxic mouse lungs revealed that HIMF deficiency restored FoxO1 nuclear retention in PVSMCs (Figure 6C). Thus, hypoxia-suppressed FoxO1 appears to be a mechanism underlying the association between autophagy and impaired BMPR2 in the context of HIMF-induced PH. Interestingly, in comparison with HMGB1/RAGE suppressors, autophagy inhibition significantly mitigated, but did not completely block, the hypoxia-induced BMPR2 dysfunction (Figure 6D). Additionally, expression of the oncoprotein kinase Pim-1 (proviral integration site for Moloney murine leukemia virus-1) was inhibited in the lungs of the HIMF KO mice (Supplemental Figure II). Given that Pim-1 is critical to PH development32 and its activation in VSMCs is RAGE-dependent,33 our data suggest the involvement of Pim-1 in HIMF-induced PH. Future studies are required to explore other HIMF/DAMP-regulated downstream factors and their interplay or feedback.
Figure 6.
HIMF signaling mediates downregulation and nuclear exclusion of FoxO1 in pulmonary vascular smooth muscle cells. A, Western blotting of lysates from mouse lung after 4 days of hypoxia (Hx). B, Quantitative analysis of data in A. Data represent means ± SEM (n = 6 per group). C, Sections of 4-day hypoxic mouse lungs were stained with FoxO1 (red) and α-smooth muscle actin (α-SMA, green). Original magnification: 400X. Photographs are representative of 4 individual lung samples per group. D, Western blot analysis of lung lysates for FoxO1 expression in mice exposed to hypoxia for 4 days and treated with HMGB1/RAGE/autophagy inhibitors. *significantly increased (p<0.05) vs the 3MA-treated group; †significantly decreased (p<0.05) vs the 3-MA-treated group. Data represent means ± SEM (n = 4-5 per group). KO, knockout; WT, wild-type.
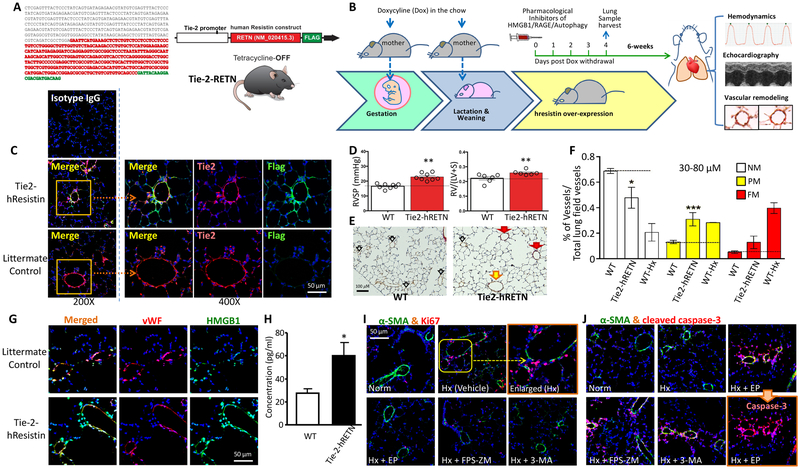
In vivo EC-restricted hresistin overexpression induces the PH phenotype in PVSMCs through HMGB1/RAGE signaling
Humans express a different analog of HIMF/RELM-α.34 To integrate human cell data into an animal study and further validate the role of the HIMF/HMGB1 signaling axis in PH, we developed the tetracycline-switchable hresistin knock-in humanized mouse line (Figure 7A and 7B). These mice exhibit spatially-controlled overexpression of recombinant hresistin [driven by the angiopoietin-2 receptor promoter (Tie-2)] in an EC-restricted manner (Figure 7A and 7C). Tie-2-hresistin transgene expression (Figure 7B and 7C) resulted in a significant pulmonary hemodynamic change, with increased RVSP (Figure 7D, left panel) and augmented right ventricular hypertrophy [assessed by Fulton index as RV/(LV+S), Figure 7D, right panel]. hResistin overexpression in ECs also led to vascular remodeling and an increase in the percentage of muscularized small vessels in lungs (Figure 7E and 7F). ELISA analysis of the BALF revealed that endothelial hresistin overexpression caused an increase in the release of alveolar HMGB1 (Figure 7H) that was associated with stronger HMGB1 signals in pulmonary ECs, as shown by an immunofluorescence assay (Figure 7G). Further pharmacologic intervention studies in these humanized mice revealed that systemic administration of inhibitors for HMGB1, RAGE, or autophagy effectively mitigated the PH proliferative phenotype in PVSMCs during the early vascular inflammatory phase (Figure 7I). Interestingly, these transgenic mice exhibited decreased activation of AMP-activated protein kinase (AMPK) in pulmonary vascular ECs (Supplemental Figure III), suggesting that hresistin-induced endothelial AMPK dysregulation also contributes to PH development. These data support the mechanistic findings of EC-PVSMC communication mediated by the HIMF-DAMP signaling axis.
Figure 7.
In vivo EC-restricted hResistin overexpression drives HMGB1/RAGE-dependent pulmonary vascular smooth muscle cell (PVSMC) proliferation and vascular remodeling during pulmonary hypertension development in mice. A, Generation of hresistin knock-in mice. Left: Nucleotide sequence for the human resistin construct. Right: schematic illustration of the Tie-2-hRETN (hresistin) transgene structure. B, Schematic of the induction of hresistin protein overexpression in endothelial cells of humanized mice. Doxycycline (Teklad Custom Diet) was administered to mothers at a dose of 2-3 mg/kg in food throughout pregnancy and during the weaning period to block hresistin expression. Dose was based on the manufacturer’s calculation that mice consume 4-5 g/day. Doxycycline was withdrawn from these mothers approximately 1 month after parturition. hResistin overexpression was induced for a total period of 6 weeks before organ harvesting and hemodynamics evaluation. C, Validation of hresistin (FLAG-tagged) expression in humanized mice. Sections were immunofluorescently stained with anti-Tie-2 (red) and anti-FLAG (green) antibodies. Signals are digitally merged in left panels. Boxed areas are enlarged and displayed along with separate channels in the right panels. Magnification: 200X (left panels) and 400X (enlarged). Representative images are from 4 individual samples per group. D, Mice with Tie-2-hresistin overexpression had higher right ventricular systolic pressure (RVSP) and Fulton index (RV/LV+S) than did the wild-type (WT) controls. **p<0.01. E, Microphotographs from lung tissue sections stained for von Willebrand factor (vWF, brown) and α-smooth muscle actin (α-SMA, red) illustrate the differences in small vessels between controls (left) and Tie2-hRETN mice (right). Red arrows mark fully muscularized vessels (FM), yellow arrows mark partially muscularized vessels (PM), and empty arrows mark non-muscularized vessels (NM). F, Percentages of FM, PM, and NM vessels in WT and Tie-2-hRETN mice. WT mice exposed to chronic hypoxia served as positive controls. *p<0.05, ***p<0.001. n = 7-8. G, Immunofluorescence images of lung tissue from Tie2-hRETN mice on day 4 after switch-on (lower panels) and from littermate controls (upper panels). Sections were stained with anti-vWF (red) and anti-HMGB1 (green) antibodies. HMGB1 signal was elevated in the vWF-positive pulmonary endothelial cells. Magnification: 400X. Representative images are from 4 individual samples per group. H, Secreted HMGB1 levels in the bronchoalveolar lavage fluid (BALF) from Tie-2-hRETN humanized mice were evaluated by ELISA (LifeSpan). Results from three separate experiments performed in duplicate are shown as mean ± SEM. *p<0.05. I and J, Humanized mice received once-daily i.p. injections of HMGB1 inhibitor (EP, 50 mg/kg), RAGE inhibitor (FPS-ZM1, 1 mg/kg), or autophagy inhibitor (3-MA, 15 mg/kg) on days 0 to 3. On day 4, mouse lungs were harvested for immunofluorescence staining of α-SMA (green) together with Ki-67 (red, I) or cleaved caspase-3 (red, J). The inhibitor-treated mice showed a decrease in proliferating PVSMCs and enhanced apoptosis of α-SMA+ cells. Original magnification: 400X. Photographs are representative of 4 individual lung samples per group.
Discussion
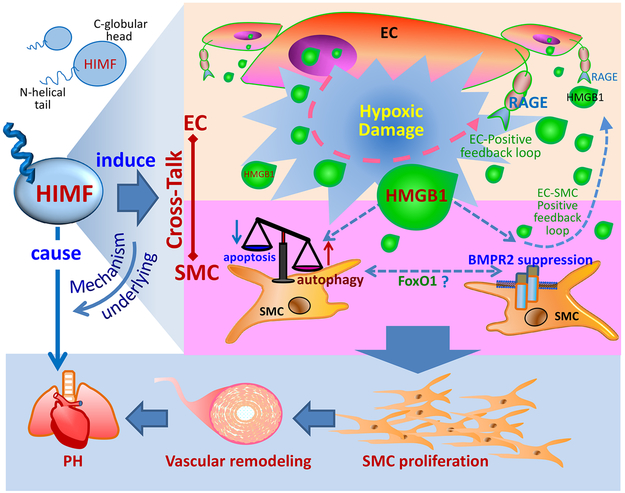
This study validated the causal role of HIMF signaling in PH and dissected the mechanism underlying HIMF-driven EC-PVSMC crosstalk required for lung vascular remodeling during the post-injury inflammatory phase (Figure 8). We demonstrated that HMGB1/RAGE signaling is critical to HIMF-initiated vascular inflammation. As a key DAMP mediator, HMGB1 produced and released by HIMF-stimulated ECs induces PVSMC proliferation in a paracrine manner to amplify the pro-PH effects of HIMF activation. The EC-derived HMGB1 may also activate RAGE in ECs and SMCs to form a positive feedback loop, resulting in the secretion and release of more HMGB1 and increased expression of RAGE7 in these pulmonary vascular cells for the perpetuation of inflammatory responses in lungs. Thus, by governing DAMPs, HIMF may trigger and continuously fuel the vascular-immune milieu of PH development.
Figure 8. Schematic illustration of the HIMF-regulated endothelial cell (EC)-smooth muscle cell (SMC) crosstalk in pulmonary hypertension (PH).
In HIMF-induced PH, HMGB1 is pivotal for mediating EC-SMC crosstalk. HIMF may trigger a self-amplification loop of DAMP signaling between ECs and SMCs. Through a paracrine mechanism, the HIMF-induced EC-derived HMGB1 favors the hyperproliferative phenotype of SMCs by regulating autophagy and BMPR2, synergistically contributing to PH pathogenesis.
EC-SMC crosstalk is the pivotal mechanism of PH pathogenesis. Our in vivo study focused on the early post-hypoxia stage to interrogate vascular interaction during PH onset. The rationale is based on our previous observation that HIMF expression is elevated by 24 hours of hypoxia in rodents and peaks at 4 days.1,2,17 HIMF itself can directly enhance PVSMC proliferation,1 and its upregulation at the early inflammation phase signifies that other synergistic effectors are activated and regulated by HIMF to perpetuate the later vascular remodeling in rodent models. During hypoxia exposure, early EC apoptosis may trigger both degenerative and reactive proliferative events required for vascular pathology in PH.3,35 We previously observed that HIMF-induced apoptotic ECs could enhance PVSMC proliferation,3 suggesting that cytokine(s) mediate the regenerative response to inflammatory tissue damage. As a DNA-binding nuclear protein and an endogenous danger signal, HMGB1 might be released from apoptotic cells after injury.4,5,36 Also the HIMF-related up- and downstream pathways, including interleukin-4, interleukin-6, SDF-1, HIF-1α, and RAGE ligand S100A11,3,37-39 all have been cited in the literature as having a close connection to HMGB1 signaling.40-42 Here, our data further indicate that HMGB1 is a master mediator of the HIMF-driven EC-SMC vascular interaction that underlies PH development.
HMGB1 released from apoptotic cells was shown to signal through RAGE.5 Our data suggest that RAGE is a pivotal DAMP receptor that mediates the HIMF/HMGB1 signaling axis and regulates the downstream Pim-1 pathway. RAGE is principally located in lungs and is being considered as a potential biomarker for therapy evaluation in PH patients.43 This DAMP receptor negatively regulates reactive oxygen species production44 and facilitates the cytoprotective effects of HMGB1 that promote cell growth.44,45 The HMGB1-RAGE pathway thus may be preferentially engaged in the HIMF-induced pathomorphologic shifts within the pulmonary vascular bed. Besides RAGE, toll-like receptor (TLR) 4 is another putative HMGB1 receptor.46 We have previously demonstrated a role for TLR4 in the HMGB1-medated angiogenic response in a mouse corneal neovascularization model.47 Additionally, Bauer et al. showed that TLR4 participates in the pro-PH activity of HMGB1.48 They also observed that right ventricular hypertrophy, but not RVSP, was attenuated in RAGE−/− mice at 3 weeks post-hypoxia.48 Another recent study10 showed that intra-tracheal delivery of RAGE siRNA caused dramatic reduction in pulmonary artery pressure and right ventricular hypertrophy in rat models of PH at 15 days after monocrotaline injection and 7 weeks after sugen/hypoxia, the time points at which PH was established.10 The use of different time points for PH measurements in the same PH animal models with the same treatment have led to conflicting conclusions.12,49 It was recently reported that hypoxia substantially increased interaction affinity between HMGB1 and RAGE, but not TLR4, in hypoxic and normoxic mouse and human PVSMCs.50 TLR4 knockdown did not significantly affect resistin-mediated monocyte migration and inflammatory cytokine production,51 suggesting that TLR4 has a less important role in HIMF/HMGB1-induced PH. However, as resistin has plausible interaction with TLR4,52 the possibility of TLR4 involvement cannot be excluded and will be clarified in our future studies. Nevertheless, our current data together with data from the literature indicate that RAGE has a critical pro-PH role, especially in HIMF-induced PH, and is the predominant receptor mediating downstream autophagy53 and BMPR2 dysfunction.10
Amplification of HIMF/HMGB1 signaling by downstream proliferation-related effectors, autophagy, and BMPR2 defects12, 27 reinforces the hyperproliferative microenvironment in PVSMCs. HMGB1 was recently identified as a pro-autophagic factor associated with its redox status and subcellular location.6, 8,53,54 Extracellular HMGB1 is a potent inducer of autophagy, which is dependent on RAGE activation to prevent oxidative injury.28,44,53,55,56 It may be that HIMF/hresistin modulates the redox biology of HMGB1 to support autophagy by activating the HIMF binding partner BTK,57 which negatively regulates reactive oxygen species production.58 HIMF/HMGB1 also dampens effects of the anti-proliferative factor BMPR2. A mechanism that links the HIMF/HMGB1-induced autophagy and BMPR2 dysfunction may be the suppression of FoxO1.12,30,31 This possibility is supported by the observation that HIMF downregulates FoxO1 expression and promotes its nuclear exclusion in PVSMCs. Thus, HIMF/HMGB1 appears to be a signaling hub of the proliferation-regulating pathway network associated with hypoxia. Profound EC death might also promote PH.3 Disruption of the HIMF/HMGB1 immune axis in our study with specific DAMP antagonists did prevent PVSMC proliferation but did not boost EC apoptosis (Figure 3). These results exclude the opposing possibility that HIMF/HMGB1 breakdown may enhance PH.
Both HIMF and its human homolog hresistin exhibit potent pro-PH properties. These rodent and human resistin-like variants, predominantly derived from immune cells in bone marrow, lung, and spleen, have a similar tissue distribution and expression pattern.34 HIMF signaling also induces recruitment of bone marrow-derived mesenchymal stem cells into the lung and their differentiation into vascular SMCs.59 This pathway could work in concert with HIMF-induced EC-SMC vascular inflammation to contribute to vascular remodeling. To more accurately mimic the hresistin/HMGB1-driven cellular crosstalk in humans, we generated the humanized mouse model that overexpresses hresistin in ECs bearing Tie-2. Notably, hresistin conditional overexpression in ECs was sufficient to cause vascular remodeling and PH development associated with elevated EC-derived HMGB1 in lungs. Knock-in of hresistin in ECs recapitulated the hypo-proliferative phenotype of PVSMCs, but this proliferative state was reversed by HMGB1/RAGE/autophagy inhibition. The hresistin-DAMP signaling may also involve AMPK dysregulation in ECs. These data confirm that HMGB1 is the linchpin for amplifying and sustaining the hresistin-evoked vascular EC-SMC interaction required for progressive remodeling. We recently verified that hresistin expression is upregulated in the lung tissue of patients with idiopathic PAH (unpublished observations). As leukocytes are the main cellular source of hresistin,34 the data also provide impetus for our ongoing study to interrogate the role of immune cells that produce HIMF/hresistin in the vascular-immune biology interface. Nevertheless, data from these Tie-2-hresistin transgenic mice validate the mechanistic findings of the HIMF-activated ECs in models that use KO animals and primary human vascular cells, and further suggest their clinical relevance.
Taken together, our data suggest that HIMF is an immune checkpoint regulator and that the HIMF/HMGB1 signaling axis plays a core role in PH. By mediating EC-SMC crosstalk, HIMF/HMGB1 modulates several downstream proliferation-related elements in PVSMCs. Recent literature suggests that suppressing HMGB1,9,60 RAGE,10 or autophagy,12 and rescuing FoxO131 or BMPR2,27 may be promising anti-PH strategies. Our study presents an option whereby comprehensively integrating the outcomes of these regimens would reverse the PH pro-proliferative microenvironment by interrupting HIMF signaling. Implementation of these findings is ongoing. The therapeutic monoclonal antibodies that we have generated to target HIMF/hresistin20 have shown promise for preventing and reversing vascular remodeling in rodent PH models (unpublished observations). We have also launched studies into the development of an hresistin biomarker for PH patients. Additional studies to dissect RELM-related complex inflammatory signaling may offer novel intervention strategies in a variety of vasculature-related lung pathologies, as well as conditions in other organs, such as cancer, atherosclerosis, and diabetes.
Supplementary Material
Highlights.
HIMF gene deficiency attenuates pulmonary hypertension (PH), which is associated with suppressed HMGB1-RAGE signaling in pulmonary endothelial cells (ECs).
HIMF/hresistin triggers HMGB1 release from ECs to induce the pro-PH phenotype of smooth muscle cells (SMCs).
HIMF/HMGB1 axis constitutes a signaling hub that regulates the proliferation-governing pathways autophagy and BMPR2 in the context of EC-SMC interaction.
EC-restricted hresistin overexpression in transgenic humanized mice drives pulmonary vascular remodeling through HMGB1-RAGE activation.
Acknowledgments:
We thank Claire F. Levine for editing this article in manuscript form.
Sources of Funding: This work was supported by National Institutes of Health (NIH) Centers for Advanced Diagnostics and Experimental Therapeutics in Lung Diseases Stage II (CADET II) 5UH2HL123827-02 and NIH 1R01HL138497-01 grants (to R.A.J.), and NIH 1R01HL135022-01A1 grant (to K.Y.-K.), as well as a Stimulating and Advancing ACCM Research (StAAR) grant (to Q.L.) from the Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University.
Nonstandard Abbreviations and Acronyms
- BALF
bronchoalveolar lavage fluid
- BMPR2
bone morphogenetic protein receptor 2
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DAMP
damage-associated molecular pattern
- EC
endothelial cell
- EP
ethyl pyruvate
- FIZZ1
found in inflammatory zone-1
- FoxO1
forkhead box O1
- HIMF
hypoxia-induced mitogenic factor
- HMGB1
high-mobility group box 1
- hresistin
human resistin
- iPAH
Idiopathic pulmonary arterial hypertension
- PH
pulmonary hypertension
- PMVEC
pulmonary microvascular endothelial cell
- PVSMC
pulmonary vascular smooth muscle cell
- RAGE
receptor for advanced glycation end products
- RELM
resistin-like molecule
- RVSP
right ventricular systolic pressure
- SMC
smooth muscle cell
- STAT3
signal transducer and activator of transcription 3
- TLR4
toll-like receptor 4
Footnotes
Disclosures: The authors have no financial conflicts of interest.
Contributor Information
Qing Lin, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD..
Chunling Fan, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD..
Jose Gomez-Arroyo, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD..
Katrien Van Raemdonck, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD..
Lucas W. Meuchel, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
John T. Skinner, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
Allen D. Everett, Division of Pediatric Cardiology, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD.
Xia Fang, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD..
Andrew A. Macdonald, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
Kazuyo Yamaji-Kegan, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD..
Roger A. Johns, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
References
- 1.Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065–1067. 10.1161/01.RES.0000073999.07698.33 [DOI] [PubMed] [Google Scholar]
- 2.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Champion HC, Crow MT, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) induces the vascular and hemodynamic changes of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L582–593. 10.1152/ajplung.90526.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaji-Kegan K, Takimoto E, Zhang A, Weiner NC, Meuchel LW, Berger AE, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (FIZZ1/RELMalpha) induces endothelial cell apoptosis and subsequent interleukin-4-dependent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;306:L1090–1103. 10.1152/ajplung.00279.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenabeele P, Vandecasteele K, Bachert C, Krysko O, Krysko DV. Immunogenic Apoptotic Cell Death and Anticancer Immunity. Adv Exp Med Biol. 2016;930:133–149. 10.1007/978-3-319-39406-0_6 [DOI] [PubMed] [Google Scholar]
- 5.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. 10.1146/annurev.immunol.021908.132603 [DOI] [PubMed] [Google Scholar]
- 6.Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ 3rd, Lotze MT. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. 10.1083/jcb.200911078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis. 2008;11:91–99. 10.1007/s10456-008-9093-5 [DOI] [PubMed] [Google Scholar]
- 8.Janko C, Filipovic M, Munoz LE, Schorn C, Schett G, Ivanovic-Burmazovic I, Herrmann M. Redox modulation of HMGB1-related signaling. Antioxid Redox Signal. 2014;20:1075–1085. 10.1089/ars.2013.5179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadamura-Takenaka Y, Ito T, Noma S, Oyama Y, Yamada S, Kawahara K, Inoue H, Maruyama I. HMGB1 promotes the development of pulmonary arterial hypertension in rats. PLoS One. 2014;9:e102482 10.1371/journal.pone.0102482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meloche J, Courchesne A, Barrier M, et al. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J Am Heart Assoc. 2013;2:e005157 10.1161/JAHA.112.005157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zabini D, Crnkovic S, Xu H, Tscherner M, Ghanim B, Klepetko W, Olschewski A, Kwapiszewska G, Marsh LM. High-mobility group box-1 induces vascular remodelling processes via c-Jun activation. J Cell Mol Med. 2015;19:1151–1161. 10.1111/jcmm.12519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res. 2013;112:1159–1170. 10.1161/CIRCRESAHA.111.300483 [DOI] [PubMed] [Google Scholar]
- 13.Guihaire J, Deuse T, Wang D, Fadel E, Reichenspurner H, Schrepfer S. Sex Differences in Immunology: More Severe Development of Experimental Pulmonary Hypertension in Male Rats Exposed to Vascular Endothelial Growth Factor Receptor Blockade. Biomed Res Int. 2015;2015:765292 10.1155/2015/765292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tofovic SP. Estrogens and development of pulmonary hypertension: interaction of estradiol metabolism and pulmonary vascular disease. J Cardiovasc Pharmacol. 2010;56:696–708. 10.1097/FJC.0b013e3181f9ea8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafikov R, Nair V, Sinari S, Babu H, Sullivan JC, Yuan JX, Desai AA, Rafikova O. Gender Difference in Damage-Mediated Signaling Contributes to Pulmonary Arterial Hypertension. Antioxid Redox Signal. 2019. 10.1089/ars.2018.7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T, Yu H, Ullenbruch M, Jin H, Ito T, Wu Z, Liu J, Phan SH. The in vivo fibrotic role of FIZZ1 in pulmonary fibrosis. PLoS One. 2014;9:e88362 10.1371/journal.pone.0088362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Poloczek A, El-Haddad H, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) in chronic hypoxia- and antigen-mediated pulmonary vascular remodeling. Respir Res. 2013;14:1 10.1186/1465-9921-14-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, Walker C, Westwick J, Thomas M. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2011;184:1171–1182. 10.1164/rccm.201103-0412OC [DOI] [PubMed] [Google Scholar]
- 19.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. 10.1161/CIRCULATIONAHA.109.927681 [DOI] [PubMed] [Google Scholar]
- 20.Lin Q, Fan C, Skinner JT, Bejia D, Van Raemdonck K, Nakahara M, Yamaji-Kegan K, RA J. Therapeutic Effects of the Generated Antibodies Targeting Human Resistin in Pulmonary Hypertension ATS (American Thoracic Society) International Conference. San Diego, CA, USA: American Journal of Respiratory and Critical Care Medicine, 2018:A7398. [Google Scholar]
- 21.Provencher S, Archer SL, Ramirez FD, Hibbert B, Paulin R, Boucherat O, Lacasse Y, Bonnet S. Standards and Methodological Rigor in Pulmonary Arterial Hypertension Preclinical and Translational Research. Circ Res. 2018;122:1021–1032. 10.1161/CIRCRESAHA.117.312579 [DOI] [PubMed] [Google Scholar]
- 22.Humbert M, Guignabert C, Bonnet S, Dorfmuller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53 10.1183/13993003.01887-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–12356. 10.1073/pnas.192222999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deane R, Singh I, Sagare AP, et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. 10.1172/JCI58642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiacco E, Castagnetti F, Bianconi V, Madaro L, De Bardi M, Nazio F, D'Amico A, Bertini E, Cecconi F, Puri PL, Latella L. Autophagy regulates satellite cell ability to regenerate normal and dystrophic muscles. Cell Death Differ. 2016;23:1839–1849. 10.1038/cdd.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. [DOI] [PubMed] [Google Scholar]
- 27.West J, Austin E, Fessel JP, Loyd J, Hamid R. Rescuing the BMPR2 signaling axis in pulmonary arterial hypertension. Drug Discov Today. 2014;19:1241–1245. 10.1016/j.drudis.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM, Krasinskas A, Lotze MT, Zeh HJ 3rd. The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc Natl Acad Sci U S A. 2012;109:7031–7036. 10.1073/pnas.1113865109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng XJ, Wu C, Yang GF, Liu QJ, Liu JX, Hao J, Xing LL, Yang M, Liu SX. TLR2 Plays a Critical Role in HMGB1-Induced Glomeruli Cell Proliferation Through the FoxO1 Signaling Pathway in Lupus Nephritis. J Interferon Cytokine Res. 2016;36:258–266. 10.1089/jir.2015.0082 [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Wang L, Yang J, Zhang P, Ma K, Zhou J, Liao W, Zhu WG. Anti-neoplastic activity of the cytosolic FoxO1 results from autophagic cell death. Autophagy. 2010;6:988–990. 10.4161/auto.6.7.13289 [DOI] [PubMed] [Google Scholar]
- 31.Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N, Grimminger F, Seeger W, Schermuly RT, Pullamsetti SS. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med. 2014;20:1289–1300. 10.1038/nm.3695 [DOI] [PubMed] [Google Scholar]
- 32.Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, Cote J, Provencher S, Sussman MA, Bonnet S. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 2011;123:1205–1215. 10.1161/CIRCULATIONAHA.110.963314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meloche J, Paulin R, Courboulin A, Lambert C, Barrier M, Bonnet P, Bisserier M, Roy M, Sussman MA, Agharazii M, Bonnet S. RAGE-dependent activation of the oncoprotein Pim1 plays a critical role in systemic vascular remodeling processes. Arterioscler Thromb Vasc Biol. 2011;31:2114–2124. 10.1161/ATVBAHA.111.230573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan C, Johns BA, Su Q, Kolosova IA, Johns RA. Choosing the right antibody for resistin-like molecule (RELM/FIZZ) family members. Histochem Cell Biol. 2013;139:605–613. 10.1007/s00418-012-1042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther. 2010;126:1–8. 10.1016/j.pharmthera.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 36.Bell CW, Jiang W, Reich CF 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–1325. 10.1152/ajpcell.00616.2005 [DOI] [PubMed] [Google Scholar]
- 37.Yamaji-Kegan K, Su Q, Angelini DJ, Champion HC, Johns RA. Hypoxia-induced mitogenic factor has proangiogenic and proinflammatory effects in the lung via VEGF and VEGF receptor-2. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1159–1168. 10.1152/ajplung.00168.2006 [DOI] [PubMed] [Google Scholar]
- 38.Johns RA, Takimoto E, Meuchel LW, Elsaigh E, Zhang A, Heller NM, Semenza GL, Yamaji-Kegan K. Hypoxia-Inducible Factor 1alpha Is a Critical Downstream Mediator for Hypoxia-Induced Mitogenic Factor (FIZZ1/RELMalpha)-Induced Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2016;36:134–144. 10.1161/ATVBAHA.115.306710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan C, Fu Z, Su Q, Angelini DJ, Van Eyk J, Johns RA. S100A11 mediates hypoxia-induced mitogenic factor (HIMF)-induced smooth muscle cell migration, vesicular exocytosis, and nuclear activation. Mol Cell Proteomics. 2011;10:M110 000901 10.1074/mcp.M110.000901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SY, Lee SW, Kim HY, Lee WS, Hong KW, Kim CD. HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1alpha activation. Eur J Immunol. 2015;45:1216–1227. 10.1002/eji.201444908 [DOI] [PubMed] [Google Scholar]
- 41.Campana L, Bosurgi L, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 for stromal cell-derived factor-1/CXCL12-dependent migration of macrophages and dendritic cells. J Leukoc Biol. 2009;86:609–615. 10.1189/jlb.0908576 [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi M, Taguchi M, Sato T, Karasawa K, Sakurai Y, Harimoto K, Ito M. Association of High-Mobility Group Box-1 With Th Cell-Related Cytokines in the Vitreous of Ocular Sarcoidosis Patients. Invest Ophthalmol Vis Sci. 2017;58:528–537. 10.1167/iovs.16-20324 [DOI] [PubMed] [Google Scholar]
- 43.Suzuki S, Nakazato K, Sugimoto K, Yoshihisa A, Yamaki T, Kunii H, Suzuki H, Saitoh S, Takeishi Y. Plasma Levels of Receptor for Advanced Glycation End-Products and High-Mobility Group Box 1 in Patients With Pulmonary Hypertension. Int Heart J. 2016;57:234–240. 10.1536/ihj.15-188 [DOI] [PubMed] [Google Scholar]
- 44.Kang R, Tang D, Lotze MT, Zeh HJ, 3rd. RAGE regulates autophagy and apoptosis following oxidative injury. Autophagy. 2011;7:442–444. [DOI] [PubMed] [Google Scholar]
- 45.Petrovic A, Bogojevic D, Korac A, Golic I, Jovanovic-Stojanov S, Martinovic V, Ivanovic-Matic S, Stevanovic J, Poznanovic G, Grigorov I. Oxidative stress-dependent contribution of HMGB1 to the interplay between apoptosis and autophagy in diabetic rat liver. J Physiol Biochem. 2017;73:511–521. 10.1007/s13105-017-0574-0 [DOI] [PubMed] [Google Scholar]
- 46.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. 10.1074/jbc.M306793200 [DOI] [PubMed] [Google Scholar]
- 47.Lin Q, Yang XP, Fang D, Ren X, Zhou H, Fang J, Liu X, Zhou S, Wen F, Yao X, Wang JM, Su SB. High-mobility group box-1 mediates toll-like receptor 4-dependent angiogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1024–1032. 10.1161/ATVBAHA.111.224048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, Bauer PM. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med. 2012;18:1509–1518. 10.2119/molmed.2012.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SJ, Smith A, Guo L, Alastalo TP, Li M, Sawada H, Liu X, Chen ZH, Ifedigbo E, Jin Y, Feghali-Bostwick C, Ryter SW, Kim HP, Rabinovitch M, Choi AM. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:649–658. 10.1164/rccm.201005-0746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia D, He Y, Zhu Q, Liu H, Zuo C, Chen G, Yu Y, Lu A. RAGE-mediated extracellular matrix proteins accumulation exacerbates HySu-induced pulmonary hypertension. Cardiovasc Res. 2017;113:586–597. 10.1093/cvr/cvx051 [DOI] [PubMed] [Google Scholar]
- 51.Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, Lee S, Kim JY, Lee J, Yang HM, Mook-Jung I, Nam KY, Chung J, Lazar MA, Kim HS. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 2014;19:484–497. 10.1016/j.cmet.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med. 2010;14:1419–1431. 10.1111/j.1582-4934.2009.00899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang R, Livesey KM, Zeh HJ 3rd, Lotze MT, Tang D. Metabolic regulation by HMGB1-mediated autophagy and mitophagy. Autophagy. 2011;7:1256–1258. 10.4161/auto.7.10.16753 [DOI] [PubMed] [Google Scholar]
- 54.Zhu X, Messer JS, Wang Y, Lin F, Cham CM, Chang J, Billiar TR, Lotze MT, Boone DL, Chang EB. Cytosolic HMGB1 controls the cellular autophagy/apoptosis checkpoint during inflammation. J Clin Invest. 2015;125:1098–1110. 10.1172/JCI76344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. 10.1038/onc.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, Bierhaus A, Lotze MT, Zeh HJ. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666–676. 10.1038/cdd.2009.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su Q, Zhou Y, Johns RA. Bruton's tyrosine kinase (BTK) is a binding partner for hypoxia induced mitogenic factor (HIMF/FIZZ1) and mediates myeloid cell chemotaxis. FASEB J. 2007;21:1376–1382. 10.1096/fj.06-6527com [DOI] [PubMed] [Google Scholar]
- 58.Honda F, Kano H, Kanegane H, Nonoyama S, Kim ES, Lee SK, Takagi M, Mizutani S, Morio T. The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nat Immunol. 2012;13:369–378. 10.1038/ni.2234 [DOI] [PubMed] [Google Scholar]
- 59.Angelini DJ, Su Q, Kolosova IA, Fan C, Skinner JT, Yamaji-Kegan K, Collector M, Sharkis SJ, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELM alpha) recruits bone marrow-derived cells to the murine pulmonary vasculature. PLoS One. 2010;5:e11251 10.1371/journal.pone.0011251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldenberg NM, Hu Y, Hu X, Volchuk A, Zhao YD, Kucherenko MM, Knosalla C, de Perrot M, Tracey KJ, Al-Abed Y, Steinberg BE, Kuebler WM. Therapeutic Targeting of High Mobility Group Box-1 in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2019. 10.1164/rccm.201808-1597LE [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.