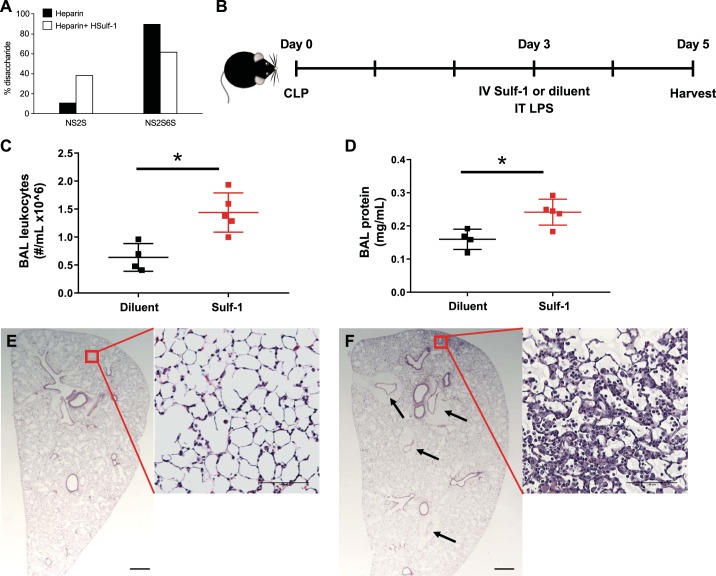
Fig. 4.
Loss of sulfatase-1 (Sulf-1) contributes to postseptic compensatory anti-inflammatory response syndrome. A: recombinant Sulf-1 was enzymatically active and efficiently removed 6-O sulfates from heparin, as shown by the decrease in trisulfated [UA(2S)-GlcNS(6S)] disaccharide content (NS2S6S) and concomitant increase of [UA-GlcNS(6S)] disulfated disaccharide (NS2S) in Sulf-1-treated heparin (white bars) compared with untreated control (black bars). B: we performed cecal ligation and puncture (CLP) on mice on day 0. On day 3, surviving postseptic mice were treated with intravenous (IV) Sulf-1 (3 μg bolus) or diluent, then challenged with intratracheal (IT) LPS (3 μg/g body wt). Animals were harvested 2 days after IT LPS. C: Sulf-1-treated animals had increased leukocytes in bronchoalveolar lavage (BAL) fluid 2 days after IT LPS, as compared with diluent treated animals 2 days after IT LPS. D: Sulf-1-treated animals also showed increased lung injury based on protein concentration in BAL fluid. E: lung histological section from a postseptic, diluent-treated animal (stained with H&E) showed very little evidence of lung inflammation. F: lung histological section from a postseptic Sulf-1-treated animal (stained with H&E) showed increased tissue consolidation 2 days after IT LPS. In addition, Sulf-1-treated animals had marked perivascular cuffs (black arrows). Scale bars on lower-magnification images = 500 μm and those on higher-magnification images = 100 μm. *P < 0.05 by Student’s t test; n = 4–5 each group.