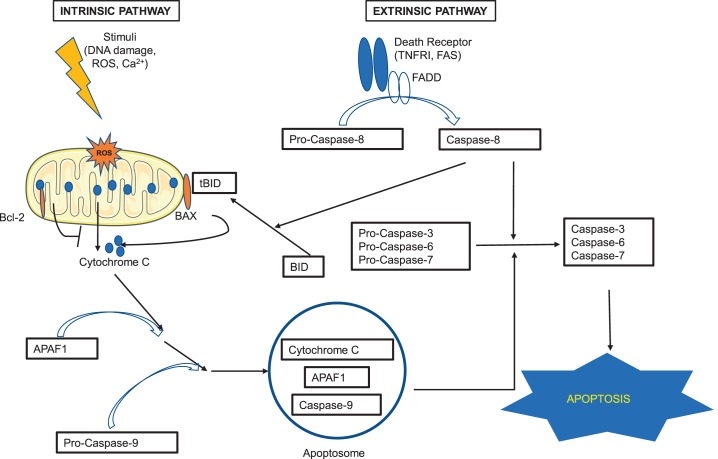
Fig. 1.
Apoptosis pathway. There are 2 major pathways that lead to apoptosis: extrinsic and intrinsic. The extrinsic pathway is triggered when ligands bind to the death receptors (such as tumor necrosis factor receptor I and Fas) on the surface of the cell. The binding induces conformational changes of the receptors that, with the help of the adaptor protein Fas-associated protein with death domain (FADD), activate pro-caspase-8 to caspase-8. The activated caspase-8 then activates pro-caspases-3,-6, and -7, the activation of which eventually leads to apoptosis. The intrinsic pathway is mitochondria dependent and happens in response to insults such as DNA damage, oxidative stress, and high calcium concentration. Activation of pro-apoptotic proteins (such as BAX) neutralizes the effects of the antiapoptotic proteins of the Bcl-2 family and leads to the release of apoptogenic factor cytochrome c from the mitochondria. Cytochrome c binds to APAF1 (apoptosis protease activating factor-1), and together they bind to and activate pro-caspase-9. The complex of cytochrome c, APAF1, and activated caspase-9 forms apoptosome, which also activates pro-caspases-3, -6, and -7, and leads to apoptosis. The cross-talk between the extrinsic and intrinsic pathways is through BH3-interacting domain (BID). When BID is cleaved by activated caspase-8, it is activated to the truncated form of BID (tBID) and translocates to mitochondria, promoting cytochrome c release. See Refs. 91 and 210.