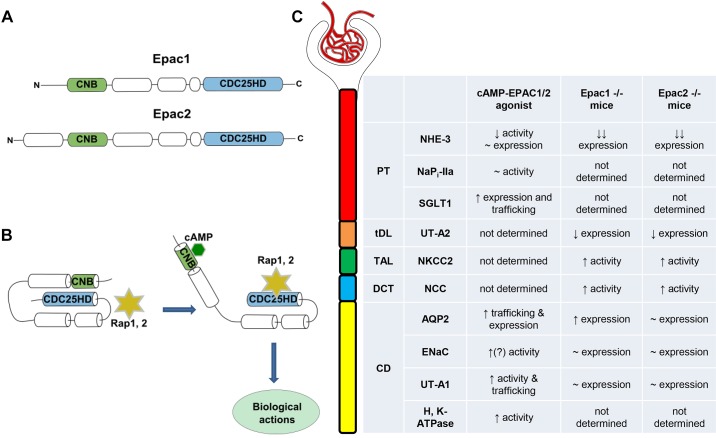
Fig. 1.
Exchange protein directly activated by cAMP (Epac) signaling in the regulation of renal tubular transport. A: an outline of major domain organization of Epac1 and Epac2 isoforms. Both isoforms contain one (for Epac1) or two (for Epac2) cyclic nucleotide binding (CNB) motif(s) at their regulatory domain at the NH2-terminus. CDC25 homology domain (CDC25-HD) is essential for interaction with Epac downstream effectors, mainly Rap1 and Rap2. B: schematic representation of Epac activation by cAMP. Binding of cAMP to the CNB motif promotes an open active Epac configuration, allowing enzymatically active CDC25-HD to interact with Rap1 and Rap2. C: summary table comparing the reported effects of pharmacological Epac stimulation and genetic deletion of Epac1 and Epac2 isoforms on the transporting systems in different segments of the renal tubule. AQP2, aquaporin 2; CD, collecting duct; DCT, distal convoluted tubule; ENaC, epithelial Na+ channel; NaPi-IIa, Na+-phosphate cotransporter type IIa; NCC, Na+-Cl− cotransporter; NHE3, Na+/H+ exchanger type 3; NKCC2, Na+-K+-2Cl− cotransporter; PT, proximal tubule; SGLT1, Na+-glucose cotransporter type 1; TAL, thick ascending limb; tDL, thin descending limb; UT-A2, urea transporter type A2; UT-A1, urea transporter type A1.