Abstract
Persistent inward current (PIC) plays a critical role in setting the gain of spinal motor neurons. In humans, most estimates of PIC are made from plantarflexor or dorsiflexor motor units in a seated position. This seated and static posture negates the task-dependent nature of the monoaminergic drive and afferent inhibition that modulate PIC activation. Our purpose was to estimate PIC during both the conventional seated posture and in a more functionally relevant anterior postural sway. We hypothesized that paired motor unit estimates of PIC would be greater when during standing compared with sitting. Soleus motor neuron PIC was estimated via the paired motor unit (PMU) technique. For each motor unit pair, difference in reference unit firing frequency (ΔF) estimates of PIC were made during isometric ramps in plantarflexion force during sitting (conventional approach) and during standing anterior postural sway (new approach). Baseline reciprocal inhibition (RI) was also measured in each posture using the poststimulus time histogram technique. ΔF estimates during standing postural sway were not different [2.64 ± 0.95 pulses/s (pps), P = 0.098] from seated PIC estimates (3.15 ± 1.45 pps) measured from the same motor unit pair. Similarly, reciprocal inhibition at the onset of each task was the same in standing (−0.60 ± 0.32, P = 0.301) and seated (−0.86 ± 0.82) postures. PMU recordings made during standing postural sway met all assumptions that underlay the PMU technique, including rate modulation ≥0.5 pps (3.11 ± 1.90 pps), rate-rate correlation r ≥ 0.7 (0.84 ± 0.13), and time between reference and test unit recruitment ≥1 s (1.83 ± 0.81 s). This study presents a novel, functionally relevant standing method for investigating PIC in humans.
NEW & NOTEWORTHY Paired motor unit (PMU) estimates of persistent inward current (PIC) in human soleus motor units are typically made in seated posture. Our study demonstrates that these estimates can be made during standing forward sway, a task that more accurately reflects the postural role of human soleus muscle. PMU recordings made during standing postural sway were validated using all previously published criteria used to test the assumptions of the PMU technique. Standing estimates of PIC did not differ from seated estimates made from the same motor unit pairs.
Keywords: intramuscular electromyography, motor neuron, paired motor unit, persistent inward current, postural sway, reciprocal inhibition
INTRODUCTION
Motor neurons are the final site of integration and modulation of the neural drive to skeletal muscle required to generate force output for postural control and movement. Initially, the motor neuron was thought to be a passive integrator. However, work over the last 40 years has revealed the important role that persistent inward currents (PICs) in motor neuron dendrites play in setting the gain of motor neurons (Heckman et al. 2008a; Hounsgaard and Kiehn 1993; Lee and Heckman 2000; Schwindt and Crill 1977). Briefly, these early studies revealed that the linear current-voltage (I-V) relationships observed in deeply anesthetized and/or acutely spinalized preparations differed from the sustained depolarizing or “plateau” potentials observed in motor neurons of lightly anesthetized preparations with intact spinal columns (Crone et al. 1988; Schwindt and Crill 1982). Furthermore, bistable firing behavior (a consequence of these long-depolarizing, voltage-dependent plateau potentials) was eliminated with acute spinalization and reinstated with administration of 5-HT (Hounsgaard et al. 1988). Subsequent studies confirmed that serotonin-induced bistability seen in earlier studies was facilitated by a plateau potential (Hounsgaard and Kiehn 1989) that is currently referred to as PIC.
PIC is a slow, depolarizing influx of positive ions (Ca2+ and Na+) into the somatodendritic region of a neuron (Heckman et al. 2008b; Schwindt and Crill 1980). Facilitated by voltage-gated L-type Ca2+ channels and persistent Na+ channels, the magnitude of this inward depolarizing current is modulated by monoamines via G protein-linked metabotropic membrane receptors (Hounsgaard and Kiehn 1993; Lee and Heckman 1999; Li et al. 2004). PIC activates near firing threshold (Bennett et al. 1998) to increase the excitability of a motor neuron and generate a greater output for a given ionotropic input (Heckman et al. 2009). Key monoaminergic inputs to motor neurons arise from serotonergic and noradrenergic brain stem nuclei that project directly to spinal motor neurons. These neuromodulatory tracts adjust motor neuron gain through the differential release of 5-HT or norepinephrine (NE). PIC has the robust effect of amplifying normalized muscle force output for a given synaptic input up to three to five times (Bennett et al. 1998; Heckman et al. 2009; Lee and Heckman 1998) such that it plays an essential (and perhaps underappreciated) role in generating motor output. It is likely that PIC serves to decrease central drive required to maintain postural muscle activation (Brownstone 2006; Heckman et al. 2009; Johnson and Heckman 2010). This is a logical assertion, given the that the predominantly slow motor units associated with antigravity musculature have the largest contribution of PIC to overall excitability (Gollnick et al. 1974). Furthermore, it has been hypothesized that PIC varies in a state-dependent and task-dependent fashion (Heckman et al. 2009; Hyngstrom et al. 2007). Increased neuromodulatory drive to the motor neuron during different arousal levels has been demonstrated indirectly through the firing of monoaminergic neurons of the brain stem nuclei (Jacobs et al. 2002), which varies between sleep states as well as in high arousal compared with resting levels (Trulson et al. 1981). Moreover, neuromodulatory drive changes with different motor tasks (Veasey et al. 1995), increasing from sitting to walking and increasing further with running (Jacobs and Fornal 1999).
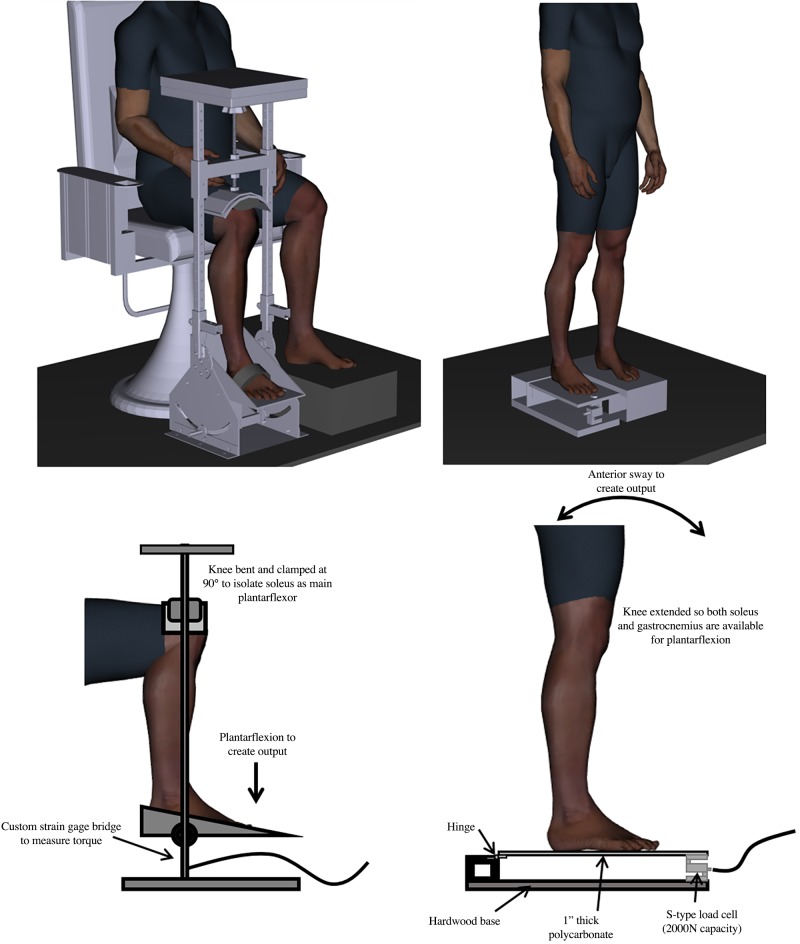
PIC is typically estimated in human motor neurons via intramuscular recordings of motor unit activity by virtue of the one-to-one relationship between action potentials fired by a neuron and each of the muscle fibers that it innervates. The paired motor unit (PMU) technique (Gorassini et al. 2004) uses the firing rate of a low-threshold “reference” motor unit as a proxy measure of synaptic input to a higher threshold “test” motor unit. Briefly, in the presence of PIC, a test motor unit will continue to fire with less synaptic input than required for recruitment. It should be noted that this technique estimates PIC in the test unit even though PIC is likely present in both the reference and test unit during the ramp contraction. Because the test unit must be recruited at least 1 s after the reference unit, it can be assumed that the reference unit would have a fully “saturated” PIC at both test unit recruitment and derecruitment. Accordingly, it can be assumed that the reference unit firing rate is a linear indicator of synaptic input to the motor neuron pool. Thus the difference between reference unit firing at test unit recruitment and derecruitment (ΔF) provides an estimate of PIC when assumptions of the PMU technique are met (Revill and Fuglevand 2011; Stephenson and Maluf 2011; Udina et al. 2010; Vandenberk and Kalmar 2014; Wienecke et al. 2009). Traditionally, estimates of PIC measurements are made from lower leg postural musculature with the participant seated and the leg positioned in an ankle dynamometer (e.g., Fig. 1, left) to record isometric plantarflexion torque. This method minimizes gastrocnemius activation by reducing the mechanical advantage of this two-joint muscle via knee flexion. Although this seated, isometric, experimental arrangement isolates the soleus muscle contribution to plantarflexion torque to yield reliable motor unit recordings, the seated contractions do not reflect the postural role of the soleus muscle during natural tasks. Therefore, the purpose of this study was to estimate soleus PIC during a functionally relevant postural task. To accomplish this, we created a novel standing protocol that uses anterior postural sway to estimate soleus PIC using PMU recordings. We hypothesized that estimates of PIC made from motor unit pairs that met all of the assumptions of the PMU technique would be greater during standing anterior postural sway than estimates of PIC made from those same motor unit pairs in the conventional seated condition.
Fig. 1.
Ankle dynamometer to measure isometric plantarflexion torque during sitting phase of experiment (left) and custom force platform to measure force during anterior-posterior sway (right). In the ankle dynamometer (left), the leg is clamped from the top of the knee (flexed at 90°) to the foot platform. A resistive transducer is used to capture isometric ankle plantarflexion torque. The ankle joint angle can be adjusted; however, it was held at 0° to agree with the standing joint angle at the beginning of the ramp. The custom force platform measured the relative force contribution of the soleus during an anterior-posterior sway. An S-type load cell (under front of platform; right) was zeroed to have feedback read zero during neutral stance. Compared with a solid-topped platform, a hinge in the rear ensured even minute adjustments in posture because forces over the front of the foot are not combatted by the tensile strength of a solidly attached platform. Participants had their foot placement marked onto paper to ensure it was in the same spot on the platform in the event of repositioning. A slight forward lean would both activate the soleus (proportional to the angle of the sway to maintain upright posture) and shift the participant’s center of pressure (exact COP not measured) forward on the platform, resulting in a greater force through the load cell. Digital human model visualization was created using Santos Pro software (SantosHuman Inc., Coralville, IA).
METHODS
Participant Recruitment
A total of 10 participants (4 men, 6 women) aged 19–25 yr (22.4 ± 1.64 yr) were recruited for this investigation, resulting in 15 useable motor unit pairs for standing and seated posture (n = 15; 6 participants had 1 useable motor unit pair, 3 participants had 2 useable motor unit pairs, 1 participant had 3 useable motor unit pairs). A target sample size of 15 motor unit pairs was reached after a three-measure, repeated-measures ANOVA sample size calculation using an effect size of f = 0.803, α = 0.05, and an assumed β = 0.95 (based on soleus ΔF data from Vandenberk and Kalmar 2014) was conducted using G*Power software (Universität Düsseldorf, Germany) (Faul et al. 2007). Recordings from nine motor unit pairs remained viable when the participants returned to standing in the final stage of the experiment. Participants had no prior history of neurological disease, recent leg injury (past 6 mo), or recent concussion (past 6 mo) and no chronic use of substances that may alter neural excitability such as nicotine, amphetamines, or selective serotonin reuptake inhibitors (SSRIs). All data were collected over a single experimental session. Before this session, participants completed an orientation to familiarize themselves with the standing postural sway task and seated isometric torque plantarflexion contractions, including maximal voluntary contractions (MVCs). This orientation also served to ensure that they were comfortable with the intramuscular EMG electrodes. The 30-min orientation served to answer any participant questions and to allow them to practice the ramp contractions such that they could perform these ramps with accuracy. This project was approved by the Wilfrid Laurier University Research Ethics Board and conforms to the Declaration of Helsinki. All participants provided written informed consent.
Experimental Protocol and Design
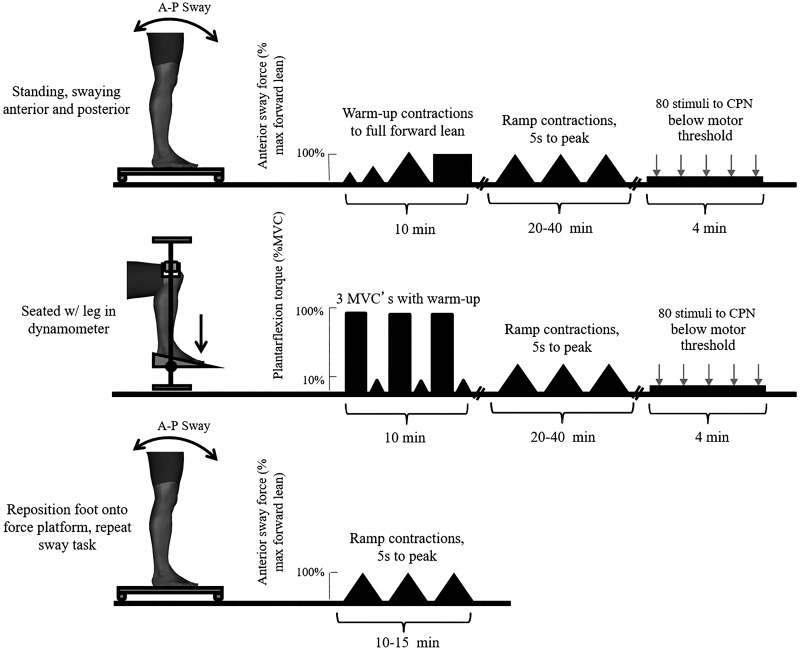
To investigate task-dependent changes in PIC, a novel standing postural sway technique was developed and compared with traditional paired motor unit estimates of PIC. After recording and stimulation electrode setup with the participant in a seated posture, the participant slowly rose to a standing posture with the right foot on the custom force platform (Fig. 1, right). The left foot was positioned on a fixed platform of equal height. Roughly 15 cm between the feet allowed for a comfortable and stable stance for all participants. The participant performed a series of standing ramp contractions followed by standing reciprocal inhibition measurement (Fig. 2, top). The participant then moved to the seated ankle dynamometer and positioned the right foot into the ankle dynamometer (Fig. 1, left). Once secured in the ankle dynamometer, the participant performed a 5-s maximal ankle plantar flexion contraction accompanied by verbal encouragement from the experimenter. Participants rested for 2 min between contractions. Once three MVCs were completed, peak force values were compared to ensure all three values were within 5% of one another. If a greater than 5% variation was detected, adequate rest was provided and participants performed another contraction until three MVCs were collected. MVC values used for analysis were the highest of the three contractions. The participant performed seated ramp contractions followed by a seated low-level continuous contraction to measure reciprocal inhibition collection (Fig. 2, middle). To ensure repeatability of measures and to demonstrate minimal intramuscular electrode shift, the participant rose again to perform a short series of standing ramp contractions (Fig. 2, bottom).
Fig. 2.
Experimental protocol. Top: participants began in a standing posture. Electrodes and intramuscular needle were positioned. Set-up was a large portion of the experiment and was always conducted methodically to ensure recording quality. Participants performed warm-up calf raises and slowly transitioned to the anterior-posterior (A-P) postural sway task. Participants were given a ramp tracing on a transparency to trace on the screen by leaning forward and back. This was repeated until 3 smooth ramps were collected. To assess reciprocal inhibition, 80 stimuli were delivered to the common peroneal nerve (CPN) during a very slight forward lean. Middle: the participant was then seated with the leg in the ankle dynamometer. Three maximal plantarflexion contractions were used for maximum voluntary contraction (MVC). Participants again performed ramp contractions, this time to 10% MVC. Reciprocal inhibition data were then collected during a low-level contraction, using 80 stimuli to the nerve to the antagonist. Bottom: participants were then transitioned back to standing for collection of one more ramp to ensure the same motor units could be followed through posture transition in both direction. Digital human model visualization was created using Santos Pro software (SantosHuman Inc., Coralville, IA).
Apparatus
To estimate PIC during isometric plantarflexion contractions in a seated position, the right leg was position in an ankle dynamometer (Marsh et al. 1981) custom built by York University Technical Department (Toronto, ON, Canada). A built-in resistive transducer measured isometric plantarflexion and dorsiflexion torque. After participants completed MVC contractions, isometric plantarflexion amplitude was displayed on a computer monitor and participants were asked to trace a triangular ramp contraction (outlined on a transparent sheet over the screen) to 10% MVC. Ramp contractions were a constant 5 s to peak and 10 s in total duration.
Data for the standing protocol were collected with the participant positioned with feet 15 cm apart, hands at their sides, and looking straight ahead. Participants were instructed to stand normally, in a comfortable posture. A custom-built force platform measured anterior posterior sway. This was achieved using a load cell (2000N S-type load cell; Interface Inc., Scottsdale, AR) under and just anterior to the toes so that a positive output voltage reflected an anterior shift in load bearing by the participant. These data provided clear biofeedback for anterior postural sway. As the participant leaned forward and an increase in pressure was placed on the forefoot, an increase in force could be seen on the screen (Fig. 3). The output of the load cell was zeroed to a resting stance when the participant was asked to maintain a comfortable stance without any forward sway. As a participant leaned forward, there was a positive deflection in the force, indicating forward sway, which returned to baseline as the participant returned to a resting stance, mimicking the conventional seated, isometric, plantarflexion ramp contractions typically used to make paired motor unit estimates of PIC. Standing peak force was designated as the most forward leaning position the participant could consistently achieve and maintain for 3 s while the heels remained in contact with the platform. This maximum forward lean was used as the benchmark for ramp height in lieu of a standing plantarflexion MVC.
Fig. 3.
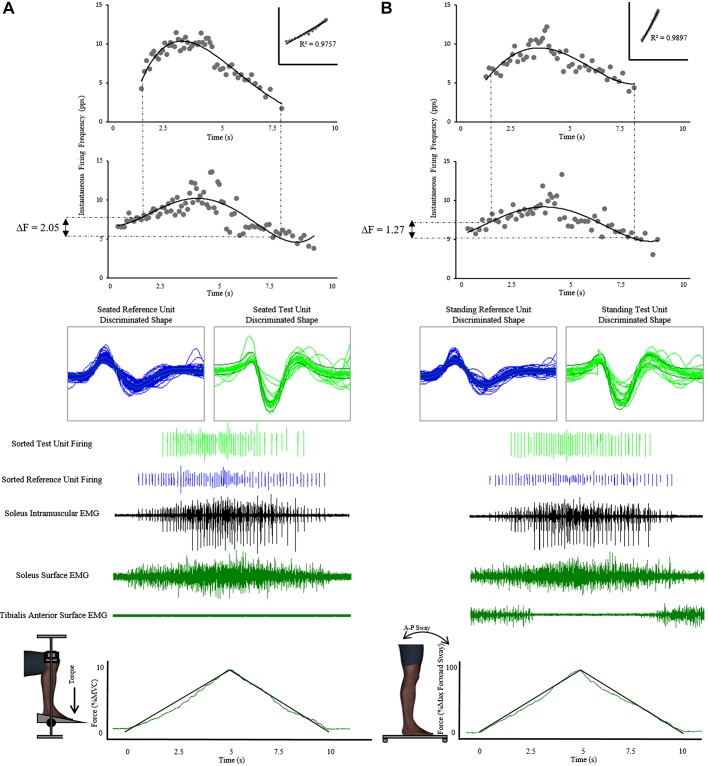
Paired motor unit (PMU) recordings and force recordings during seated and postural sway ramp tasks. Similarly to the isometric plantarflexion ramps in the seated position (A, bottom, force tracing task using plantarflexion), standing ramp templates were provided over the screen on a transparency for participants (B, bottom, force tracing task using forward lean). Peak ramp force was normalized to the most comfortable anterior lean position at which the participant could maintain consistent force output. Participants were instructed to position their weight over the heels during the non-sway phase, and this value of force output was zeroed. Participants had multiple practice attempts to ensure reliable ramps could be produced during the experimental protocol. Conventional PMU recordings are made in a seated posture (A). This has always provided investigators with reliable, repeatable measures but is divergent from what likely happens during upright standing posture. To obtain PMU estimates of persistent inward current (PIC) during standing posture, a standing forward sway protocol was designed. In the current study, isometric plantarflexion torque (seated technique) was replaced with an anterior postural way. This force was quantified using a load cell placed under the anterior portion of the custom platform where the participants stood. The standing electromyography (EMG) tracings and sorted unit traces (B, middle) show consistent firing rates, unit shapes, and recruitment patterns to seated EMG recordings (A, middle), demonstrating that a standing postural sway protocol is capable of obtaining PMU recordings. PMU plots (top graphs, test unit above reference unit) for both seated (A) and standing (B) protocols resemble recordings obtained by past investigations. Furthermore, standing estimates also meet all PMU technique criteria, further validating standing collection of differences in reference unit firing frequency (ΔF; top graphs, rate-rate correlation inset). Digital human model visualization was created using Santos Pro software (SantosHuman Inc., Coralville, IA). A-P, anterior-posterior; MVC, maximum voluntary contraction; pps, pulses/s.
Data Acquisition
Data acquisition and analysis were completed using Spike2 software (version 7.02; CED Limited, Cambridge, UK). Analog-to-digital conversion and sequencing of electrical stimuli were carried out through a 64-bit Micro1401-3 unit (CED Limited, Cambridge, UK).
Intramuscular EMG.
Single-motor unit recordings were obtained using 50.8-μm Formvar-insulated stainless steel wires (California Fine Wire Company, Grover Beach, CA). Three wires were inserted into the lateral aspect of the soleus (2 cm distal to the inferior border of the gastrocnemius lateral head as determined by muscle palpation) on the right leg using a 27-gauge BD PrecisionGlide needle (Becton Dickinson Company, Franklin Lakes, NJ). Intramuscular electrode wires and hypodermic needles were autoclaved together as a unit while wrapped in aluminum foil with the needles capped. Typical of delicate, wrapped utensils, a pre-vacuum cycle with a 20-min sterilization time at 250°C and a 20-min passive drying time was used. The electrode was secured using an ~2-mm hooked end on the wires, which provided stability through basic movements but was easily removed on experiment completion. To complete the electrode setup, the wires were input into a 10× preamplifier (EQ Inc., Chalfont, PA) and secured to the leg with an adhesive pad.
Surface EMG.
Ag-AgCl electrodes epoxy-embedded with a 10× preamplifier (EQ Inc., Chalfont, PA) were positioned over the tibialis anterior (TA) and lateral soleus. Electrodes had recording surfaces of 0.5 cm2 and an interelectrode distance of 1.2 cm. Preamplifiers input to a custom-built, variable gain, second-stage amplifier (York University Machine Shop, Toronto, ON, Canada). A ground was placed on the proximal medial tibia. All skin contacts were shaved and cleaned using scrubbing alcohol pads, and electroconductive gel was applied to contacts to enhance the electrode-skin interface. Intramuscular EMG signals were sampled at 20,000 Hz, with all other surface EMG inputs sampled at 2,000 Hz. Force was sampled at 150 Hz from both the force platform and the ankle dynamometer. Online analog filtering of intramuscular signals was performed using a NeuroLog system (NL106 amplifier insert, NL126 filter insert; Digitimer Ltd., Welwyn Garden City, UK). A bandpass filter was applied to intramuscular recordings, attenuating signal outside a 200- to 3,000-Hz range. Low-end cutoff was altered slightly to optimize signals during each experimental session. After collection, surface EMG data were high-pass filtered with a corner edge cutoff frequency of 20 Hz (as recommended by De Luca et al. 2010, and Winter et al. 1980), force data were low-pass filtered online at 50 Hz, and all data were subjected to an online 60-Hz notch filter.
Nerve stimulation.
A 2.5-cm2 carbonized rubber stimulation electrode was positioned over the common peroneal nerve, just lateral to the head of the fibula to activate the nerve to the antagonist, when reciprocal inhibition of the soleus was measured. A Digitimer constant-current stimulator (model DS7AH; Digitimer Inc., Welwyn Garden City, UK) was used to deliver stimuli for reciprocal inhibition quantification. Stimulation to elicit reciprocal inhibition was set at 80% of soleus motor threshold (defined as the stimulus intensity needed to elicit a >50-μV response tibialis anterior M wave for at least 50% of stimulations). Threshold for standing was reassessed during a comfortable standing position. All pulses were 1 ms in duration.
Paired Motor Unit Technique for PIC Estimation
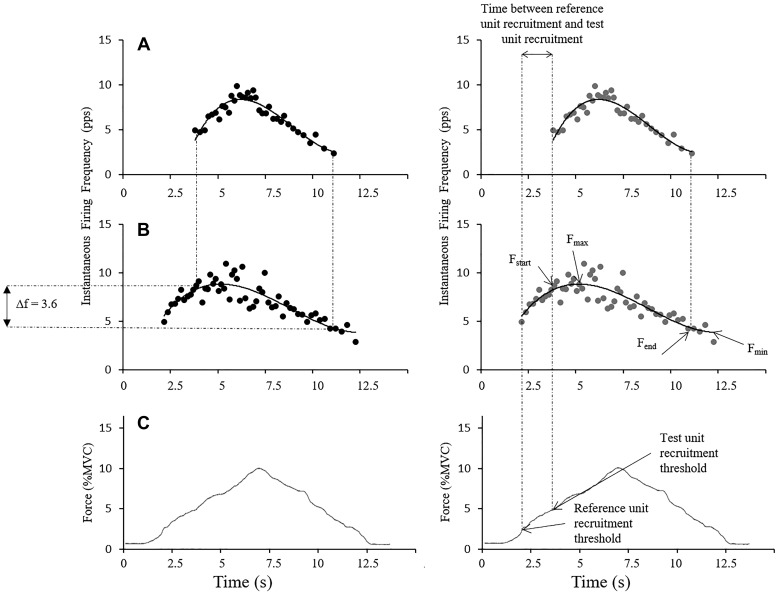
The paired motor unit technique used to estimate PIC in this investigation requires a comparison of the firing rates of two motor units from the ramp contraction. The first unit to be recruited (lower threshold) is the reference unit. A second, higher threshold unit is the test unit (Fig. 3). Single-motor unit recordings were sorted online by a spike-sorting algorithm using Spike2 software (version 7.02; CED Limited, Cambridge, UK). Recordings were filtered offline with a hum-remove filter (Spike2 script; CED Limited, Cambridge, UK). This filter decomposed repetitive sequences of oscillating baseline noise to improve the signal to noise ratio and offline spike discrimination. Spikes were sorted on the basis of amplitude and shape, fit to templates for each active motor unit, and then confirmed with manual inspection and sorting of the spike data as needed. For example, if two units fired near simultaneously, the two spike waveforms would summate and fail to fit the template for those two motor units. In this case, a marker denoting a spike was manually inserted to replace the missing spike marker for that motor unit. Instantaneous firing rate was plotted for both units over the duration of the ramp. Plots were fitted with a fourth-order polynomial curve to obtain smoothed firing frequency for any given time in the ramp. Estimation of PIC was obtained by calculating the difference in reference unit firing frequency (ΔF) at test unit recruitment and derecruitment (Fig. 4).
Fig. 4.
Calculation of ΔF and rate modulation, where ΔF is calculated as the difference in reference unit firing frequency between test unit recruitment and test unit derecruitment (A and B, left), and rate modulation is calculated as the difference between the range of reference unit firing range. Peak reference unit firing frequency (Fmax; B, right) to the last calculated firing frequency of the reference unit (Fmin; B, right) is quantified and compared with the calculated ΔF value (Fstart − Fend; B, right) for that motor unit pair. If ΔF is within 0.5 pulses/s (pps) of the rate modulation, the pair does not meet the assumption that the reference unit is a sensitive indicator of net excitatory input to the motor neuron for the duration of the ramp contraction. The relative force production (percent maximum voluntary contraction, %MVC) that accompanies the firing rates plotted in A and B is shown in C (same for both calculations).
Poststimulus Time Histogram Technique for Reciprocal Inhibition
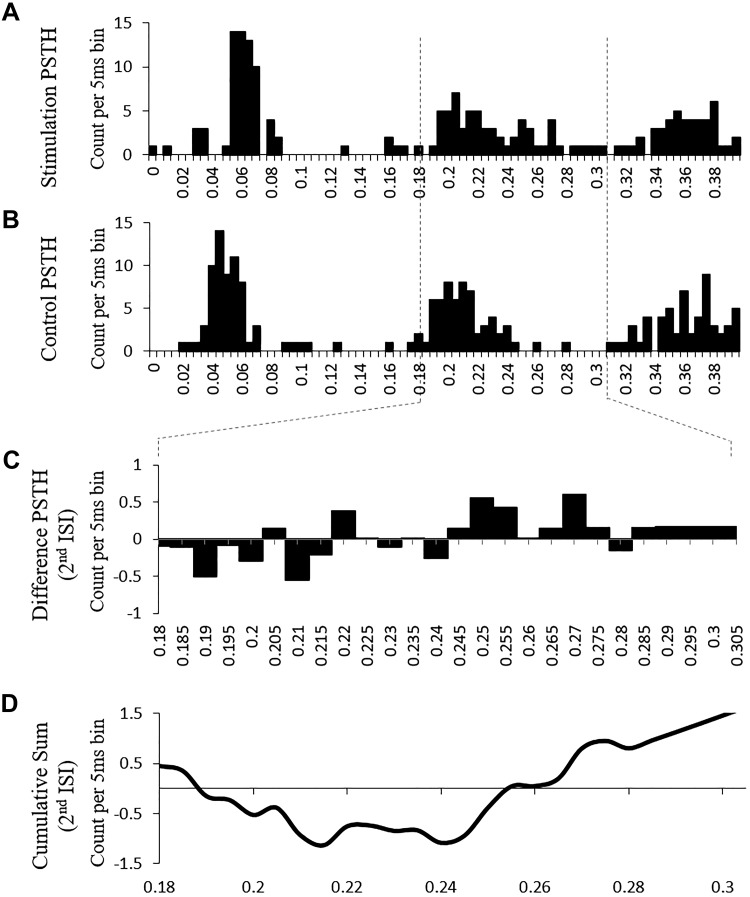
Reciprocal inhibition of each reference motor unit was estimated using the poststimulus time histogram (PSTH) technique (Aymard et al. 1995) (Fig. 5). Electrical stimulation of the common peroneal nerve (CPN) activated the reciprocal inhibitory pathway at the axons of Ia afferent sensory neurons during a sustained low-level contraction in which the participant had only one or two active units visible on the intramuscular EMG recordings. Once a constant firing rate of the reference soleus motor unit (matched shape and amplitude to of the reference unit recorded during the ramps) was established, a sequencing script was used to elicit soleus spike-triggered stimulation of the common peroneal nerve approximately. The sequence was set up to deliver pulses to the CPN ~80 ms before a soleus motor unit firing to optimize reciprocal inhibition of the soleus motor unit. For example, if the reference unit was firing at 8 Hz such that the interspike interval was 125 ms, the stimulus was delivered 45 ms after the triggering spike to arrive ~80 ms ahead of the next anticipated spike. Eighty sub-motor threshold stimuli were delivered over 4 min of the constant low-level plantarflexion contraction to create a stimulation PSTH (Fig. 5A). For control values, 80 control markers were inserted into the data 80 ms before the next expected spike to create a control PSTH (Fig. 5B). A PSTH was used to quantify the difference in firing times between spikes during normal firing (control) and those preceded by the stimulation to the CPN for a span of up to 0.4 s using 5-ms bins. A difference histogram was created from the control PSTH and stimulation PSTH (Fig. 5C). A cumulative sum of this difference histogram was then plotted with a larger deviation below the x-axis, denoting larger inhibition (Fig. 5D). This investigation complied with previous investigations and employed the use of the peak negative value in the 180- to 305-ms range to identify inhibition during the second interspike interval (Vandenberk and Kalmar 2014).
Fig. 5.
The cumulative sum poststimulus time histogram (PSTH) technique was used to quantify reciprocal inhibition. Sample data are shown from 1 participant. A: stimulation PSTH. The common peroneal nerve (CPN) was stimulated to activate the reciprocal inhibition pathway. The number of spikes (count) were plotted in 5-ms bins for 400 ms after each stimulus. B: control PSTH. This PSTH reflects the interspike intervals (ISI) during soleus motor unit activity without stimulation of the nerve to the antagonist (i.e., CPN). C: difference PSTH for 2nd ISI. Spike counts from the control PSTH were subtracted from the stimulation PSTH to quantify reciprocal inhibition. A negative value signifies inhibition. D: cumulative sum of 2nd ISI. Difference PSTH counts were cumulatively added. This cumulative sum is used to detect changes from the mean and the timing of these changes. A negative deflection indicates inhibition, and the amount of inhibition was quantified by the peak negative deflection value.
Assumptions of the Paired Motor Unit Technique
The assertion that ΔF provides an estimate of PIC rests on a number of assumptions. We tested these assumptions for each motor unit pair that was included in statistical analyses. It was critical in this investigation to examine all previously published criteria to determine whether ΔF estimates of PIC made during standing postural sway could be considered valid.
The first major assumption of the technique is that there is a shared, common synaptic drive to both the reference and the test unit. Several studies report motor unit pair rate-rate correlations to validate the shared drive assumption (Gorassini et al. 2004; Powers et al. 2008; Stephenson and Maluf 2011; Udina et al. 2010). The rate-rate correlation coefficient provides a measure of common synaptic modulation between two concurrently active motor units. This coefficient was calculated by plotting averaged instantaneous firing frequency in 200-ms bins (Powers et al. 2008) for both reference and test units for the duration of the ramp. Mean firing frequency values were correlated to obtain a Pearson’s r correlation coefficient. A minimum value of r = 0.7 or r2 = 0.5 is considered sufficient evidence of common synaptic drive to the reference and test units. Accordingly, in the present study all ramps involving motor unit pairs with r2 < 0.5 were excluded from statistical analysis.
Paired motor unit analysis also assumes full activation of the reference unit PIC at the time of test unit recruitment such that the reference unit provides a linear estimate of synaptic drive. PIC is a long-lasting depolarization; however, it also has a relatively slow activation (Bennett et al. 2001) and may take up to 2 s to become fully saturated (Udina et al. 2010). As such, reference unit-to-test unit recruitment intervals <1 s have poor validity (Stephenson and Maluf 2011), likely with shorter durations leading to smaller, and sometimes negative, ΔF values. This investigation ensured that only motor unit pairs with recruitment intervals >1 s were analyzed.
In addition, the paired motor unit technique assumes that the reference motor unit firing rate is sensitive enough to detect changes in the net excitatory input. In other words, increased synaptic input to the motor neuron should result in an increase in its firing rate. Thus rate modulation of the reference unit throughout the ramp contraction must exceed the ΔF estimate of PIC for that estimate to be valid. Rate modulation is calculated as the difference between the range of reference unit firing range (Fmax − Fmin on Fig. 4) and ΔF for that motor unit pair (Stephenson and Maluf 2011), and motor unit pairs with a reference unit rate modulation value within 0.5 pulses/s (pps) of ΔF are excluded from analysis (Stephenson and Maluf 2011). Similar to the previously mentioned assumption that reference unit firing frequency can only be a linear indicator of excitability once PIC is fully developed, ΔF estimates of PIC are only accurate if the test unit PIC is also fully developed. Accordingly, a minimum of 2 s of test unit activity is recommended to assert with reasonable certainty that ΔF is an accurate estimate of test unit PIC (Stephenson and Maluf 2011; Udina et al. 2010). As such, only test units with a minimum of 2 s of consistent firing were included in analysis.
It is possible that the ΔF value derived from paired motor unit recordings is only partially due to PIC and may be the result of other nonlinear firing properties of motor neurons. First shown in a simulation study (Revill and Fuglevand 2011), and later in humans (Vandenberk and Kalmar 2014), motor neuron properties such as spike-frequency adaptation (SFA) and spike-threshold accommodation (STA) are likely to contribute to ΔF in a manner that is dependent on the parameters of the ramp contractions. Specifically, longer ramp durations increase the extent to which SFA contributes to ΔF (Revill and Fuglevand 2011; Sawczuk et al. 1995; Vandenberk and Kalmar 2014). Where SFA is a time-dependent phenomenon, STA contributes to ΔF in ramps that are performed with a slower rate of rise (Bradley and Somjen 1961; Revill and Fuglevand 2011; Schlue et al. 1974; Vandenberk and Kalmar 2014; Wigton and Brink 1944). Both computer simulation and in vivo human paired motor unit recordings would suggest that ramp rate of rise should be ~2% MVC/s and total ramp duration should be no more than 10 s to minimize the contribution of STA and SFA to ΔF (Revill and Fuglevand 2011; Vandenberk and Kalmar 2014).
Beyond the ramp criteria needed to satisfy assumptions of the paired motor unit technique are some other aspects of muscle activation and motor unit firing characteristics that could have varied between postures. Because ΔF is positively correlated with muscle activation at test unit recruitment (Stephenson and Maluf 2011), soleus muscle activation (root mean square amplitude) was measured over the 500-ms period following test unit recruitment in both the seated and standing ramps. These values were normalized to 500 ms of soleus surface EMG during an MVC to provide an estimate of muscle activation at both test and reference unit recruitments between standing and seated postures.
Statistical Analyses
Differences between initial standing collection and seated collection were analyzed using two-tailed paired-samples t tests. Reciprocal inhibition was only measured at two time points (standing and seated) and was therefore evaluated using paired-samples t tests. Finally, a bivariate correlation between ΔF and RI was conducted only for those participants who demonstrated reciprocal inhibition. Paired-samples t test results are reported after the referenced comparison as “(t value [df], P value).”
To examine repeatability of the standing protocol, all available return-to-standing data were analyzed using repeated-measures (standing, seated, return to standing) ANOVAs. All ANOVAs with significant main effects were examined using pairwise comparisons and no confidence interval adjustments for multiple comparisons (least significant difference) for post hoc analysis. All statistical tests had an alpha level set at 0.05. Where test data showed a significant result in Mauchly’s test, failing to reject the null hypothesis of assumed sphericity, ANOVA degrees of freedom (df) were corrected using a Greenhouse-Geisser correction to limit the potential for type 1 error. Repeated-measures ANOVA test results are reported after the referenced comparison as “(F value, [dfgroup, dferror], P value, η2).”
RESULTS
Assumptions of the Paired Motor Unit Technique
All motor unit pairs had a test unit activation ≥1 s after reference unit recruitment, suggesting that PIC was fully activated in the reference unit. Seated measurements had significantly longer time between reference unit recruitment and test unit recruitment (t = −5.460 [14], P < 0.001; Table 1). Pearson correlations for each motor unit pair met the r2 ≥ 0.5 (r > 0.7) requirement (Table 1), providing evidence that the reference and test units share a common level of synaptic drive. Rate-rate correlations were not significantly different between postures (t = 0.086 [14], P < 0.933). Rate modulation of reference units (Fmax − Fmin) for each ramp was consistently more than 0.5 pps greater than ΔF (Table 1) and did not significantly differ between standing and seated measures (t = −0.196 [14], P < 0.848). Finally, duration of ramp rise (t = −1.886 [14], P < 0.080) and decline (t = −0.066 [14], P < 0.949) (Table 1) did not differ between postures and corresponded with previously recommended ramp parameter guidelines to minimize the contributing effects of other intrinsic motor neuron properties that could contribute to nonlinear firing (Revill and Fuglevand 2011; Vandenberk and Kalmar 2014). We also examined possible correlations of ΔF to time between reference unit recruitment and test unit recruitment (the proxy measure of PIC saturation). There was no significant correlation between ΔF and time between reference unit recruitment and test unit recruitment in standing (r = −0.340, P = 0.905) or seated measures (r = −0.184, P = 0.512).
Table 1.
Paired motor unit validity criteria and ramp characteristics for seated and standing measures
| Posture |
|||
|---|---|---|---|
| Standing | Seated | Return to Standing | |
| Requirements for motor unit pairs | |||
| Time between reference and test unit recruitment, s (minimum accepted value: >1 s,b >2 sa,c) |
1.83 ± 0.81d | 2.79 ± 1.20d | 2.56 ± 1.24 |
| Rate modulation (minimum difference of 0.5 ppsa,c) |
3.11 ± 1.90 | 3.21 ± 1.44 | 2.44 ± 1.15 |
| Rate-rate correlation coefficient (minimum accepted value: r ≥ 0.7a,c) |
0.84 ± 0.13 | 0.84 ± 0.11 | 0.80 ± 0.15 |
| Ramp and firing rate characteristics | |||
| Duration of ramp rise, s | 5.51 ± 0.37 | 5.91 ± 0.73 | 5.57 ± 0.48 |
| Duration of ramp decline, s | 5.96 ± 0.61 | 5.97 ± 0.58 | 5.60 ± 0.81 |
| Instantaneous firing frequency of reference unit at recruitment, pps | |||
| Only first ISI | 5.14 ± 0.84 | 5.36 ± 1.37 | 5.45 ± 2.79 |
| Average of first 3 ISIs | 6.23 ± 0.99 | 6.01 ± 1.12 | 5.98 ± 1.07 |
| Instantaneous firing frequency of test unit at recruitment, pps | |||
| Only first ISI | 5.39 ± 1.61 | 5.92 ± 2.36 | 5.28 ± 1.35 |
| Average of first 3 ISIs | 6.21 ± 1.43 | 6.41 ± 1.20 | 7.21 ± 2.68 |
| Control unit firing frequency at test unit recruitment, pps | 8.59 ± 1.67 | 9.50 ± 1.68 | 9.68 ± 1.25 |
| Control unit firing frequency at test unit derecruitment, pps | 5.95 ± 1.49 | 6.35 ± 1.77 | 6.63 ± 1.56 |
| Antagonist and soleus muscle activation | |||
| Relative antagonist muscle activation at reference unit recruitment, %baseline TA sEMG RMS | 69.32 ± 26.41d,e | 14.70 ± 6.79d,e | 83.15 ± 48.14e |
| Relative antagonist muscle activation at test unit recruitment, %baseline TA sEMG RMS | 34.92 ± 25.35d | 14.98 ± 8.16d | 31.96 ± 31.88 |
| Relative soleus muscle activation at reference unit recruitment, %MVC soleus sEMG RMS | 5.51 ± 3.40d | 3.27 ± 2.79d | 5.46 ± 5.62 |
| Relative soleus muscle activation at test unit recruitment, %MVC soleus sEMG RMS | 17.70 ± 15.04d | 7.46 ± 4.43d | 20.45 ± 10.68 |
Values are group means ± SD in participants at standing (n = 15), seated (n = 15), and return to standing (n = 9) positions. All motor unit pairs accepted for analysis in this experiment exceeded the minimum accepted value for published validation criteria:
Instantaneous firing frequencies (pulses/s, pps) at recruitment of test or reference units were quite low when only the first interspike interval (ISI) was isolated.
Significant difference between seated and standing posture (paired-samples t test; n = 15).
Main effect of posture (RM ANOVA with least significant difference pairwise comparisons; n = 9).
MVC, maximum voluntary contraction; sEMG RMS, root mean square of surface electromyography; TA, tibialis anterior.
Persistent Inward Current and Reciprocal Inhibition
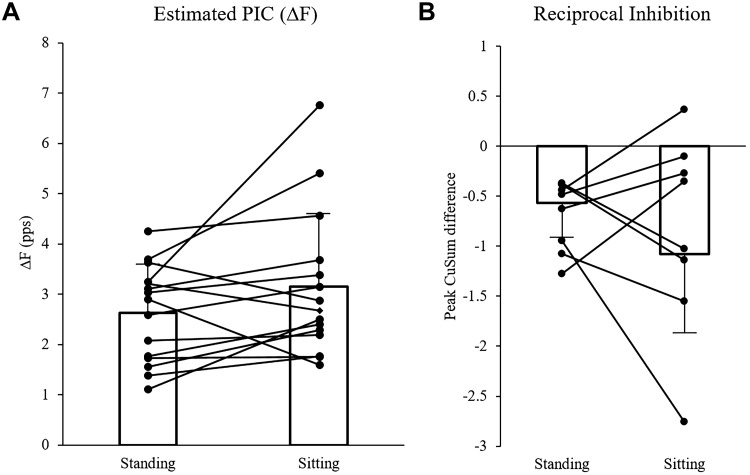
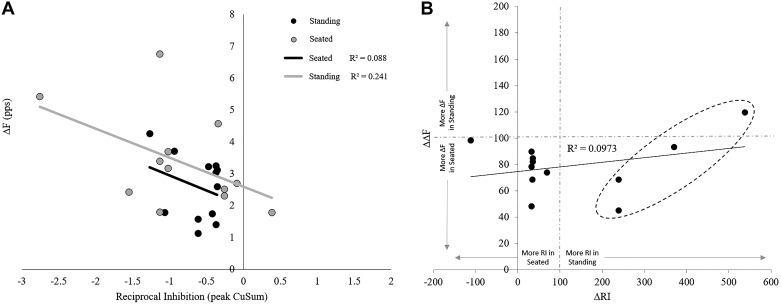
There was no effect of posture on ΔF estimates of PIC. No difference was found between postures (t = −1.771 [14], P = 0.098), with mean standing ΔF measurements of 2.64 ± 0.95 pps and seated estimates of 3.15 ± 1.45 pps. Mean and overlaid individual ΔF data can be seen in Fig. 6A. Figure 6B also depicts the mean and overlaid individual data for reciprocal inhibition between postures. Cumulative sum (CuSum) estimates of RI in the standing posture (−0.60 ± 0.32) were not different (t = 1.086 [11], P = 0.301) from estimates in the seated position (−0.86 ± 0.82). There was no relationship between ΔF and CuSum estimates of RI in the standing posture (r = −0.296, P = 0.349; Fig. 7A) or in the seated posture (r = −0.491, P = 0.105; Fig. 7A). Pearson analysis of the difference in ΔF between standing and seated postures (ΔΔF) and the difference in reciprocal inhibition between standing and seated (ΔRI) was not significantly correlated (r = 0.257, P = 0.419; Fig. 7B). A post hoc power analysis performed using G*Power software (Universität Düsseldorf, Germany) on the ΔF t test shows an effect size of 0.455 and power of 0.375 (Faul et al. 2007).
Fig. 6.
Differences in reference unit firing frequency (ΔF) and baseline reciprocal inhibition (RI). A: ΔF values (pulses/s, pps) did not differ between standing postural sway and seated plantarflexion. B: similarly, baseline RI did not differ between standing and seated postures. Note that a larger negative value denotes increased reciprocal inhibition. Individual values are represented by circles, and the connecting line denotes two values obtained from the same participant. PIC, persistent inward current.
Fig. 7.
Correlation between baseline reciprocal inhibition (RI) and subsequent firing frequency difference (ΔF) estimates of persistent inward current (PIC) during ramps. A: a decrease in PIC is expected with increased RI (more negative values). This investigation replicated this relationship between RI and estimates of PIC in seated posture (r = 0.557, P < 0.05). However, this relationship did not persist to the new standing PIC methodology (r = −0.366). B: graph depicts a correlation between the difference in ΔF (ΔΔF) and the difference in RI (ΔRI). ΔΔF is the change in ΔF during standing measurements as a function of traditional seated measurements. Thus an increase on the y-axis above 100 indicates standing ΔF greater than seated. Likewise, ΔRI is the change in baseline RI (PSTH deflection) during standing measurements as a function of traditional seated measurements, and an increase on the x-axis above 100 indicates standing RI greater than seated. There was no correlation between ΔΔF and ΔRI, demonstrating no uniform relationship between PIC and RI in the transition from seated to standing. However, there is an interesting trend indicated by data points within the dashed circle: these units had greater RI during standing measurements and show a mild positive trend of greater RI in standing with greater ΔF during postural sway. We noted a potential difference in strategy with respect to agonist-antagonist cocontraction, and potentially this is seen in the ΔΔF-ΔRI relationship as well.
Effect of Posture on Motor Unit Recruitment and Muscle Activation
There was not a significant difference in reference unit firing frequency at recruitment between standing and seated postures, as measured with only the first interspike interval (t = −0.488 [14], P = 0.633) or using an average from the first three interspike intervals (t = 0.665 [14], P = 0.517; Table 1). Similarly, test unit firing frequency at recruitment was not significantly different between postures when measured using the first interspike interval (t = −1.150 [14], P = 0.270) or when estimated using the average of the first three interspike intervals (t = −0.622 [14], P = 0.544; Table 1). Reference unit firing frequency at test unit recruitment did not significantly differ between standing and seated postures (t = −1.652 [14], P = 0.121), nor did reference unit firing frequency at test unit derecruitment (t = −0.665 [14], P = 0.517; Table 1).
Soleus muscle activation (%MVC) was significantly greater during standing compared with seated measurements at reference unit recruitment (t = 2.813 [11], P = 0.017; Table 1) and at test unit recruitment (t = 2.447 [11], P = 0.032). TA muscle activation at ramp start was not significantly different between postures (t = 0.060 [14], P = 0.953) but was significantly greater in standing at reference unit recruitment (t = 8.472 [14], P < 0.001) and at test unit recruitment (t = 3.422 [14], P = 0.004) in both standing measurements than in the seated posture (Table 1).
Repeated-Measures Analysis
Repeated-measures analysis was limited by n = 9 motor unit pairs collected in the return to standing condition, although it does provide insight into the repeatability and robustness of the new standing protocol across a full set of experimental conditions. There was no effect of posture on ΔF estimates of PIC (F = 0.577 [2, 16], P = 0.180, η2 = 0.481). There was no significant difference in reference unit firing frequency at recruitment between standing and seated postures, as measured with only the first interspike interval (F = 0.58 [2, 16], P = 0.855, η2 = 0.019) or using an average from the first three interspike intervals (F = 1.450 [2, 16], P = 0.264, η2 = 0.153). Test unit firing frequency at recruitment did not differ between postures when measured using the first interspike interval (Greenhouse-Geisser correction, F = 1.079 [1.24, 9.88], P = 0.341, η2 = 119) or when estimated using the average of the first three interspike intervals (F = 1.259 [2, 16], P = 0.311, η2 = 0.136). Between postures, reference unit firing frequency at test unit recruitment did not significantly differ (F = 0.546 [2, 16], P = 0.590, η2 = 0.036), nor did reference unit firing frequency at test unit derecruitment (F = 0.300 [2, 16], P = 0.745, η2 = 0.036). Agonist muscle activation (soleus) was not significantly different between postures at reference unit recruitment (F = 1.515 [2, 14], P = 0.254, η2 = 0.178) or at test unit recruitment (Greenhouse-Geisser correction, F = 6.499 [1.18, 8.27], P = 0.300, η2 = 0.481). TA muscle activation at both ramp start (F = 0.088 [2, 14], P = 0.916, η2 = 0.012) and test unit recruitment (F = 2.384 [2, 14], P = 0.119, η2 = 0.254) was not significantly different between postures; however, TA activation at reference unit recruitment was higher (F = 12.396 [2, 14], P = 0.001, η2 = 0.639) in both standing measurements than in the seated posture. A post hoc power analysis performed using G*Power software (Universität Düsseldorf, Germany) on the ΔF × posture ANOVA shows an effect size of 0.489 and power of 0.399 (Faul et al. 2007).
Paired Motor Unit Technique Variability
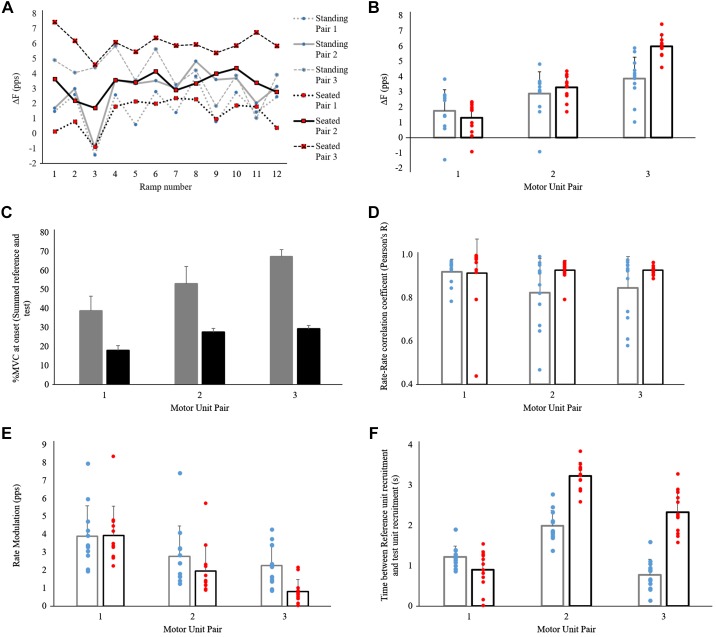
In one participant, we were able to record three motor unit pairs across 12 successful ramp contractions. We used these data to conduct one additional post hoc analysis of reliability and variability of the paired motor unit technique (Fig. 8). No data have been excluded due to inability to meet minimum criterion validity measures. Standing PIC estimates (ΔF) increased in order of test unit recruitment (pair 1, 1.77 ± 1.38 pps; pair 2, 2.88 ± 1.44 pps; pair 3, 3.88 ± 1.39 pps). Seated ΔF also increased in order of unit recruitment at a rate that was lower compared with that in standing but consistent across motor unit pairs (Fig. 8B; pair 1, 1.30 ± 1.03 pps; pair 2, 3.29 ± 0.78 pps; pair 3, 5.99 ± 0.71 pps). Rate-rate correlation coefficients were similar in standing (pair 1, 0.92 ± 0.06; pair 2, 0.82 ± 0.16; pair 3, 0.85 ± 0.15) and seated measurements (pair 1, 0.91 ± 0.16; pair 2, 0.93 ± 0.05; pair 3, 0.93 ± 0.02), with slightly more overall variability during the standing condition. Rate modulation decreased with motor unit pair but was also quite variable in both standing (pair 1, 3.89 ± 1.70 pps; pair 2, 2.77 ± 1.70 pps; pair 3, 2.26 ± 1.16 pps) and seated postures (pair 1, 3.93 ± 1.64 pps; pair 2, 1.95 ± 1.41 pps; pair 3, 0.82 ± 0.66 pps). Time between reference unit recruitment and test unit recruitment was highly variable from ramp to ramp in both standing (pair 1, 1.22 ± 0.27 s; pair 2, 1.99 ± 0.38 s; pair 3, 0.77 ± 0.39 s) and seated postures (pair 1, 0.90 ± 0.47 s; pair 2, 3.23 ± 0.34 s; pair 3, 2.33 ± 0.53 s) despite consistent rates of ramp rise (standing, 5.41 ± 0.47 s; seated, 5.47 ± 0.34 s) and decline (standing, 5.57 ± 0.45 s; seated, 5.39 ± 0.78 s).
Fig. 8.
Within-participant variations in paired motor unit estimates of persistent inward current (PIC) and validity measures. Data were obtained from a single participant who had multiple ramp force profiles (n = 12) with low error and clear, useable single-motor unit recordings. A: differences in reference unit firing frequency at test unit recruitment and derecruitment (ΔF) over time demonstrate consistently variable data [standing: pair 1 SD = 1.38 pulses/s (pps), pair 2 SD = 1.44 pps, pair 3, SD = 1.39 pps; seated: pair 1 SD = 1.03 pps; pair 2 SD = 0.78 pps; pair 3 SD = 0.71 pps] in both standing and seated measures as well as within a motor unit pair ramp-over-ramp. B: mean ΔF values grouped by posture show a clear trend of increasing ΔF with motor unit pair. Both standing (blue) and seated (red) postures show largely linear increases with motor unit pairs (1, 2, 3). C: correlation between ΔF and test unit recruitment threshold for both standing (r = 0.461, P = 0.005) and seated (r = 0.760, P < 0.001) data in this participant is consistent with previously reported positive correlations. D: rate-rate correlation coefficients remained relatively unchanged when effects of posture or motor unit pair were compared. E: rate modulation showed a trend of linear decrease with motor unit pair number in both standing and seated postures. F: finally, time between reference unit and test unit recruitment is shown to have large variations both by posture and by motor unit pair. Although a common analysis technique is to utilize the earliest recruited test unit to meet a minimum time between recruitment of >1 s, these data suggest that use of a later recruited test unit increases the average time between recruitment, ensuring greater PIC saturation of the reference unit.
DISCUSSION
Although the role that PIC may play in movement and postural tasks is postulated and discussed in several reviews (Heckman et al. 2005, 2009; Johnson et al. 2012), PIC has not been estimated in a functionally relevant standing posture in humans. This study demonstrates that PMU recordings made during standing anterior postural sway meet the assumptions of the PMU technique. However, we were unable to determine whether there were differences between seated and standing measures of recruitment patterns and firing rate modulation with this small sample of motor unit pairs. We hypothesized that if valid PMU recordings could be made during standing anterior postural sway, then ΔF estimates of PIC made from standing PMU recordings would be greater than seated measures due to an expected increase in neuromodulatory drive to motor neurons during a standing postural task. Although direct measurement of increased neuromodulatory drive during standing is not possible in humans, several studies indirectly support this claim. Research has shown firing rate changes in the deep brain stem nuclei from which neuromodulatory projections originate, increasing with increases in arousal (Trulson et al. 1981), varying across motor tasks (Veasey et al. 1995), and increasing with rate of locomotion (Jacobs and Fornal 1999). We expected that ΔF estimates of PIC would be significantly greater during standing postural sway than during seated isometric plantarflexion. With this underpowered sample, we did not find that standing estimates of PIC differed from seated estimates made from the same motor unit pairs.
As suggested in several prominent reviews (ElBasiouny et al. 2010; Heckman et al. 2008a; Johnson and Heckman 2010), not only is PIC essential to functional movement, but resultant self-sustained firing of motor neurons in the absence of descending synaptic input would be advantageous to the antigravity role of the postural muscles (Hounsgaard et al. 1988). As Heckman et al. (2009) explain, Ia and vestibulospinal systems alone are capable of generating only 5–6 nA (Heckman and Binder 1991), or 1–3% of maximum muscle force. This activation falls far short of generating the current needed to maintain the 5–10% MVC soleus activation needed to maintain posture (Walmsley et al. 1978), again demonstrating the integral role of PIC to adjust motor neuron gain to meet force output demands. However, in the present study, mean ΔF values were nearly identical in standing and seated posture. It should be noted that there was a large range in ΔΔF (the difference between seated and standing ΔF for each motor unit pair) between participants, with individual standing ΔF ranging from 45% to 180% of seated ΔF. Nonetheless, although the majority of motor unit pairs demonstrated little deviation in ΔF between postures, the motor unit pairs that did exhibit the large ΔΔF values, had much greater ΔF in the seated measures than in standing postural sway, which was contrary to our hypothesis.
We expected (but did not have a sufficient sample size to detect) a difference in PIC between the two tasks because we assumed that neuromodulatory drive would increase in the standing posture. However, PIC is also modulated by inhibitory input that may differ between the two tasks. We hypothesized that there would be less inhibition of the soleus in the standing posture; however, there was no difference between PSTH estimates of RI made during quiet standing (−0.60 ± 0.32) compared with the seated measurement (−0.86 ± 0.82). One of the limitations in this study was that it was not possible to measure reciprocal inhibition during the task and that the ankle was not maintained in a neutral position during the standing measure of RI. To generate a PSTH in standing, the participant was asked to sway forward slightly and hold the position that activated the reference soleus motor unit at a target firing rate. In contrast, the reference motor unit was activated via an isometric contraction in the seated measurement of RI, resulting in very little movement about the ankle in the ankle dynamometer (Marsh et al. 1981). During the standing contractions, we would expect the TA to exert less inhibition of the soleus motor neuron pool during the ascending limb of the ramp (shifting the center of gravity in an anterior direction relative to the base of support) as the TA muscle length decreased (Granit 1958; Grillner and Udo 1971). Conversely, during the descending phase (shifting the center of gravity back to resting position), we would expect reciprocal inhibitory input to soleus motor units as the TA lengthens. Although several investigations report reductions in PIC with increased inhibitory input in human motor neurons (Revill and Fuglevand 2011, 2017; Vandenberk and Kalmar 2014), Revill and Fuglevand (2017) demonstrate that the effect of inhibition is greatest during the ascending phase of ramp contractions. In that study, inhibitory input via sural nerve stimulation resulted in linearization of tibialis anterior motor neuron firing rates during the ascending limb of an isometric dorsiflexion contraction, but not the descending limb. In our study, any expected increases in RI due to TA stretch during standing sway would have been more likely to occur during the descending phase of the contractions, when soleus motor neuron firing rates are already linear and less affected by inhibitory input from the TA.
It is possible that participants used different strategies to trace the force ramps. Although the standing task resulted in significantly more TA activation than the seated task (Table 1), some participants appear to have used tibialis anterior activation to moderate fluctuating soleus activation during both standing and seated protocols. On inspection, this was more prominent during the ascending phase of ramps (Fig. 9, left) than during the final, derecruitment portions (Fig. 9, right). It has already been observed that cocontraction is a common strategy employed to stabilize postural sway (Katz et al. 1988; Nielsen and Kagamihara 1992). Thus variable levels of cocontraction between participants may account for differences in reciprocal inhibition, presynaptic inhibition, and possibly PIC. This finding does not demystify why a null result was measured.
Fig. 9.
Participant force output strategy changes on ramp rise and decline. Left: percent tibialis anterior (%TA) activation (cocontraction) 2.5 s into the ramp contraction reveals 2 distinct force output strategies. Although there is no significant correlation, differences in reference unit firing frequency (ΔF) estimated from motor unit pairs in the top cluster are associated with more TA cocontraction around the time of soleus reference and test unit recruitment. Right: there is no correlation between %TA activation (cocontraction) and ΔF estimates of persistent inward current (PIC) during the descending limb of the ramp, 2.5 s before the contraction ends (left).
Although we were unable to detect an effect of task (seated isometric vs. standing postural sway) on estimates of PIC with this small sample, we have demonstrated that estimates of PIC in soleus motor units can be made during a more functionally relevant task than the conventional seated, isometric plantarflexion contractions typically used with the paired motor unit technique (Fig. 3). Even when the well-established conventional approach is used, there is some debate as to whether ΔF provides a true estimate of PIC (Revill and Fuglevand 2011; Vandenberk and Kalmar 2014; Wienecke et al. 2009). Over the last decade, a number of criteria have been established to determine whether selected motor unit pairs meet the assumptions that underpin the use of paired motor unit recordings to estimate PIC in humans. To utilize the firing frequency of a single motor unit to estimate descending ionotropic drive to the motor neuron pool, the PMU technique requires the control unit to have a fully saturated firing rate, meaning this unit is no longer experiencing frequency-current nonlinearities or “warm-up.” The accepted experimental criteria requires a reference unit to be active for >1 s before test unit recruitment to validate the assumption of descending drive (Gorassini et al. 2004). Firing rate correlations can validate the assumption that both the reference and test unit are receiving equal descending drive. Experimentally, a Pearson’s r coefficient of r2 > 0.5 signifies a sufficient equality in assumed descending drive (Gorassini et al. 2004; Powers et al. 2008). Additional validity criteria have been added since inception of the PMU technique, primarily, the use of rate modulation measurement to ensure that the reference unit continues to be a sensitive indicator of descending drive throughout the course of the ramp contraction (Fmax − Fmin > 0.5 pps) (Stephenson and Maluf 2011). Finally, ramp contraction rates of rise (~2% MVC/s) (Revill and Fuglevand 2011; Vandenberk and Kalmar 2014), and total contraction duration (10-s total duration, 5 s to peak and 0-s plateau) (Revill and Fuglevand 2011; Vandenberk and Kalmar 2014) must also be monitored to limit confounding output measure ΔF from firing rate implications of other time-sensitive intrinsic properties.
We sought to determine whether ΔF estimates of PIC derived from paired motor unit recordings during a functional and dynamic postural sway task would meet the previously published criteria for the validity of seated paired motor unit estimates of PIC (Gorassini et al. 2004; Revill and Fuglevand 2011; Stephenson and Maluf 2011). We found that 1) control motor units were likely to have saturated PICs before recruitment of the test motor unit (there was a minimum of 1 s between control and test unit recruitment), 2) control and test motor units appear to share a common motor drive, and 3) the control motor unit remained a sensitive indicator of changes in synaptic drive (rate modulation of the reference unit was always >0.5 pps of ΔF). In other words, all previously published validation criteria were met for each motor unit pair in both seated and standing postural sway estimates of PIC.
One final possibility for error is the possible absence of PIC saturation in the reference units during standing measurements. This investigation followed recommendations that a minimum of 1 s should separate recruitment of reference and test units (Bennett et al. 2001; Powers et al. 2008); however, other recommendations have called for a separation of 2 s or more to allow for PIC saturation of the reference unit (Gorassini et al. 2004; Stephenson and Maluf 2011). One possibility for the lack of ΔF difference measured between postures could be that recruitment orders vary between the isometric seated task and the dynamic and functional standing sway task. During voluntary isometric contractions, soleus motor unit recruitment closely resembles the size principle recruitment pattern (Nardone et al. 1989), but we have already discussed how isometric experimental conditions lack generalizability to functional movement. Although there is some debate, evidence exists that eccentric soleus contractions can result in a reversal of size principle recruitment pattern (Howell et al. 1995; Nardone et al. 1989). This could possibly shorten the time between recruitments during the ascending limb of the postural sway-induced ramp contractions, especially during any minimal increase in velocity of the lengthening contraction (Pasquet et al. 2006). However, there was no significant correlation between ΔF and time between reference unit recruitment and test unit recruitment in the standing postural sway ramps (r = −0.340, P = 0.905). Furthermore, although not every unit met the more stringent 2-s criteria for time between reference and test unit recruitment, average times were well over 1 s (standing: 1.83 ± 0.81 s, P = 0.905; seated: 2.79 ± 1.20 s; return to standing: 2.56 ± 1.24 s), and it is likely that PIC was saturated.
One participant in this study performed the ramp contractions particularly well and completed 12 ramps in each of the seated and standing conditions that closely matched the template and that yielded three different motor unit pairs. Although it was not our a priori objective, this unique data set from one participant afforded us the opportunity to 1) examine the ramp-to-ramp variability in ΔF for ramps that meet all published criteria for valid paired motor estimates of PIC, 2) to examine the ramp-to-ramp variability in these inclusion criteria, and 3) to report the variability between motor unit pairs recruited at different recruitment thresholds during the same contraction. Several interesting trends emerged that may direct future investigations. First, in this participant, ΔF was extremely variable from ramp to ramp, changing as much as 2 pps (up to 30% of typically reported ΔF measurements) despite the fact that all ramps closely matched the target force template. Ideally, one would expect ΔF values and measures of paired motor unit validity within a single participant, in the same posture and with consistent ramp rate of rise and duration, to be highly reproducible; however, this is not the case. This preliminary finding warrants a more systematic investigation to assess within-participant reproducibility of paired motor estimates of PIC. Second, ΔF increased in a linear fashion when plotted in relation to test unit recruitment threshold (%MVC; Fig. 8C). This finding is similar to a previous analysis of the paired motor unit technique in both humans (Stephenson and Maluf 2011) and the decerebrate cat (Powers et al. 2008).
We used only motor unit pairs that met all previously published validation criteria and followed each pair to make repeated measures of ΔF from the same motor unit pairs in both seated and standing postures. Previously, this repeated-measures approach with a similar sample size of 14 motor units revealed significant differences in ΔF estimates of PIC with different ramp parameters (1 − β = 0.958) (Vandenberk and Kalmar 2014). Furthermore, Udina et al. (2010) demonstrated a significant effect of amphetamine on ΔF estimates of PIC with two separate, similarly sized samples of motor units (16 motor unit pairs in the drug condition and 11 motor unit pairs in the placebo condition). However, post hoc power calculation in the present study revealed that our sample size of 15 motor unit pairs did not result in the power required to reject a false null hypothesis (1 − β = 0.375). It seems likely that statistical power of previous studies with roughly the same sample sizes (Udina et al. 2010; Vandenberk and Kalmar 2014) is driven by larger effect sizes than in the current study (e.g., 0.455 in the present study compared with 0.803 in Vandenberk and Kalmar 2014). Thus, in the present study, any differences in monoaminergic drive that might result in differences in PIC between the postures may simply not be large enough to overcome the inherent variability of PMU estimates of ΔF. We hope that the present study might direct future work by demonstrating that paired motor unit recordings that meet published criteria can be made during a more functionally relevant postural task. It is our hope that future work, employing new strategies that increase the yield of acceptable motor unit pairs, will result in more powerful studies of task- and state-dependent modulation of PIC.
Conclusion
In conclusion, this investigation did not reveal any difference in human soleus PIC between a seated measurement and standing postural sway. The novel finding in this study is that ΔF estimates of PIC can be made in the standing position from motor unit pairs that meet all of the assumptions of the conventional, seated, paired motor unit technique. This is significant because standing postural sway represents a more functionally relevant motor task for antigravity muscles of the lower limb than seated isometric contractions. Accordingly, estimates of PIC in motor units of antigravity musculature that are made in a standing posture may provide greater insight into the modulation of PIC in aging, injury, fatigue, and other factors that may alter the neural control of movement. Finally, although it was not our original objective, we do present considerable within-participant ramp-to-ramp variability in paired motor unit estimates of PIC, which clearly warrants further investigation.
GRANTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC RGPIN-2015-05438).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.C.A.F. and J.M.K. conceived and designed research; R.C.A.F. performed experiments; R.C.A.F. analyzed data; R.C.A.F. and J.M.K. interpreted results of experiments; R.C.A.F. and J.M.K. prepared figures; R.C.A.F. and J.M.K. drafted manuscript; R.C.A.F. and J.M.K. edited and revised manuscript; J.M.K. approved final version of manuscript.
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at https://doi.org/10.5281/zenodo.3362567. These materials are not a part of this manuscript, and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
ACKNOWLEDGMENTS
We acknowledge and thank Dr. Stephen D. Perry, Manfred Gartner, and Jeff Rice for work designing and building the postural sway platform, as well as Michael S. Vandenberk for technical assistance and contributions to study design.
Present address of R. C. A. Foley: Kinesiology Department, Faculty of Health Sciences, The University of Ontario Institute of Technology, 2000 Simcoe St. North, Oshawa, ON, Canada L1H 7K4.
REFERENCES
- Aymard C, Chia L, Katz R, Lafitte C, Pénicaud A. Reciprocal inhibition between wrist flexors and extensors in man: a new set of interneurones? J Physiol 487: 221–235, 1995. doi: 10.1113/jphysiol.1995.sp020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- Bradley K, Somjen GG. Accommodation in motoneurones of the rat and the cat. J Physiol 156: 75–92, 1961. doi: 10.1113/jphysiol.1961.sp006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM. Beginning at the end: repetitive firing properties in the final common pathway. Prog Neurobiol 78: 156–172, 2006. doi: 10.1016/j.pneurobio.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Kiehn O, Mazieres L, Wigström H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J Physiol 405: 321–343, 1988. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Gilmore LD, Kuznetsov M, Roy SH. Filtering the surface EMG signal: Movement artifact and baseline noise contamination. J Biomech 43: 1573–1579, 2010. doi: 10.1016/j.jbiomech.2010.01.027. [DOI] [PubMed] [Google Scholar]
- ElBasiouny SM, Schuster JE, Heckman CJ. Persistent inward currents in spinal motoneurons: important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis. Clin Neurophysiol 121: 1669–1679, 2010. doi: 10.1016/j.clinph.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Sjödin B, Karlsson J, Jansson E, Saltin B. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch 348: 247–255, 1974. doi: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Granit R. Neuromuscular interaction in postural tone of the cat’s isometric soleus muscle. J Physiol 143: 387–402, 1958. doi: 10.1113/jphysiol.1958.sp006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Udo M. Motor unit activity and stiffness of the contracting muscle fibres in the tonic stretch reflex. Acta Physiol Scand 81: 422–424, 1971. doi: 10.1111/j.1748-1716.1971.tb04916.x. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulation of the steady-state input-output function of the cat medial gastrocnemius motoneuron pool. J Neurophysiol 65: 952–967, 1991. doi: 10.1152/jn.1991.65.4.952. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Hyngstrom AS, Johnson MD. Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol 586: 1225–1231, 2008a. doi: 10.1113/jphysiol.2007.145078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14: 264–275, 2008b. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J. Motoneuron excitability: the importance of neuromodulatory inputs. Clin Neurophysiol 120: 2040–2054, 2009. doi: 10.1016/j.clinph.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 405: 345–367, 1988. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol 414: 265–282, 1989. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol 468: 245–259, 1993. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JN, Fuglevand AJ, Walsh ML, Bigland-Ritchie B. Motor unit activity during isometric and concentric-eccentric contractions of the human first dorsal interosseus muscle. J Neurophysiol 74: 901–904, 1995. doi: 10.1152/jn.1995.74.2.901. [DOI] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ. Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci 10: 363–369, 2007. doi: 10.1038/nn1852. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology 21, Suppl: 9S–15S, 1999. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martín-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40: 45–52, 2002. doi: 10.1016/S0165-0173(02)00187-X. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Heckman CJ. Interactions between focused synaptic inputs and diffuse neuromodulation in the spinal cord. Ann N Y Acad Sci 1198: 35–41, 2010. doi: 10.1111/j.1749-6632.2010.05430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Hyngstrom AS, Manuel M, Heckman CJ. Push-pull control of motor output. J Neurosci 32: 4592–4599, 2012. doi: 10.1523/JNEUROSCI.4709-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of Ia fibres in man while standing. Brain 111: 417–437, 1988. doi: 10.1093/brain/111.2.417. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic α1 agonist methoxamine. J Neurophysiol 81: 2164–2174, 1999. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol 91: 2236–2246, 2004. doi: 10.1152/jn.01010.2003. [DOI] [PubMed] [Google Scholar]
- Marsh E, Sale D, McComas AJ, Quinlan J. Influence of joint position on ankle dorsiflexion in humans. J Appl Physiol 51: 160–167, 1981. doi: 10.1152/jappl.1981.51.1.160. [DOI] [PubMed] [Google Scholar]
- Nardone A, Romanò C, Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol 409: 451–471, 1989. doi: 10.1113/jphysiol.1989.sp017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. J Physiol 456: 373–391, 1992. doi: 10.1113/jphysiol.1992.sp019341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquet B, Carpentier A, Duchateau J. Specific modulation of motor unit discharge for a similar change in fascicle length during shortening and lengthening contractions in humans. J Physiol 577: 753–765, 2006. doi: 10.1113/jphysiol.2006.117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC. Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol 100: 292–303, 2008. doi: 10.1152/jn.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revill AL, Fuglevand AJ. Effects of persistent inward currents, accommodation, and adaptation on motor unit behavior: a simulation study. J Neurophysiol 106: 1467–1479, 2011. doi: 10.1152/jn.00419.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revill AL, Fuglevand AJ. Inhibition linearizes firing rate responses in human motor units: implications for the role of persistent inward currents. J Physiol 595: 179–191, 2017. doi: 10.1113/JP272823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Spike frequency adaptation studied in hypoglossal motoneurons of the rat. J Neurophysiol 73: 1799–1810, 1995. doi: 10.1152/jn.1995.73.5.1799. [DOI] [PubMed] [Google Scholar]
- Schlue WR, Richter DW, Mauritz KH, Nacimiento AC. Accommodation of cat spinal motoneurones to linearly rising currents before and during long-term changes of membrane potential. Brain Res 76: 213–221, 1974. doi: 10.1016/0006-8993(74)90455-7. [DOI] [PubMed] [Google Scholar]
- Schwindt P, Crill WE. A persistent negative resistance in cat lumbar motoneurons. Brain Res 120: 173–178, 1977. doi: 10.1016/0006-8993(77)90510-8. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol 43: 1700–1724, 1980. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol 48: 875–890, 1982. doi: 10.1152/jn.1982.48.4.875. [DOI] [PubMed] [Google Scholar]
- Stephenson JL, Maluf KS. Dependence of the paired motor unit analysis on motor unit discharge characteristics in the human tibialis anterior muscle. J Neurosci Methods 198: 84–92, 2011. doi: 10.1016/j.jneumeth.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL, Morrison AR. Raphe unit activity during REM sleep in normal cats and in pontine lesioned cats displaying REM sleep without atonia. Brain Res 226: 75–91, 1981. doi: 10.1016/0006-8993(81)91084-2. [DOI] [PubMed] [Google Scholar]
- Udina E, D’Amico J, Bergquist AJ, Gorassini MA. Amphetamine increases persistent inward currents in human motoneurons estimated from paired motor-unit activity. J Neurophysiol 103: 1295–1303, 2010. doi: 10.1152/jn.00734.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberk MS, Kalmar JM. An evaluation of paired motor unit estimates of persistent inward current in human motoneurons. J Neurophysiol 111: 1877–1884, 2014. doi: 10.1152/jn.00469.2013. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15: 5346–5359, 1995. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol 41: 1203–1216, 1978. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Wienecke J, Zhang M, Hultborn H. A prolongation of the postspike afterhyperpolarization following spike trains can partly explain the lower firing rates at derecruitment than those at recruitment. J Neurophysiol 102: 3698–3710, 2009. doi: 10.1152/jn.90995.2008. [DOI] [PubMed] [Google Scholar]
- Wigton RS, Brink F Jr. Studies of accommodation of nerve in parathyroid deficiency. J Clin Invest 23: 898–903, 1944. doi: 10.1172/JCI101564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA, Rau G, Kadefors R, Broman H, De Luca CJ. Units, Terms and Standards in the Reporting of EMG Research. A Report of the Ad Hoc Committee of the International Society of Electrophysiological Kinesiology. Baltimore, MD: International Society of Electrical Kinesiology, 1980. [Google Scholar]