Abstract
Among the 12 P-type ATPases encoded by the genome of Mycobacterium tuberculosis(Mtb), CtpF responds to the greatest number of stress conditions, including oxidative stress, hypoxia, and infection. CtpF is the mycobacterial homolog of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) of higher eukaryotes. Its expression is regulated by the global regulator of latency, DosR. However, the role that CtpF plays in the mycobacterial plasma membrane remains unknown. In this study, different functional analyses showed that CtpF is associated with calcium pumping from mycobacterial cells. Specifically, Mtb CtpF expression in Mycobacterium smegmatis cells prevents Ca2+ accumulation compared with wild type (WT) cells. In addition, plasma membrane vesicles from recombinant membranes, in which the direction of ion transport is inverted, accumulate more Ca2+ compared with vesicles obtained from the WT strain. This findings support the hypothesis that CtpF contributes to calcium efflux from mycobacterial cells. Accordingly, Mtb cells defective in ctpF (MtbΔctpF) accumulate more Ca2+ compared with WT cells, while the Ca2+-dependent ATPase activity is significantly lower in the mutant cells. Interestingly, the deletion of ctpF in Mtb impairs the tolerance of the bacteria to oxidative and nitrosative stress. Overall, our results indicate that CtpF is associated with calcium pumping from mycobacterial cells and the response to oxidative stress.
Keywords: Bacteria, Biochemistry, Biomolecules, Microbiology, Microorganism, Bioinformatics, Mycobacterium tuberculosis, Mycobacterium smegmatis, P-type ATPases, Plasma membrane, CtpF, Ca2+ efflux
Bacteria; Biochemistry; Biomolecules; Microbiology; Microorganism; Bioinformatics; Mycobacterium tuberculosis; Mycobacterium smegmatis; P-type ATPases; Plasma membrane; CtpF; Ca2+ efflux.
1. Introduction
Tuberculosis (TB) is produced by the acid-fast bacillus Mtb and is one of the top 10 causes of death worldwide. In 2017, there were 6.4 million new cases reported and 1.6 million deaths by TB [1]. The incidence of TB has increased as a result of the emergence of multidrug and extensively resistant (MDR and XDR) mycobacterial strains, Mtb-HIV coinfection, and the ineffectiveness of the Bacillus Calmette–Guérin (BCG) vaccine [1, 2]. Therefore, the search for alternative control strategies is a priority that relies on a better understanding of the molecular mechanism used by Mtb to succeed as intracellular pathogen. In this sense, the role played by cell membrane proteins and transporters in the mycobacterial interaction with the host cell environment is pivotal. Previous studies have suggested the relevance of P-type ATPases in the mycobacterial physiology and host-pathogen interaction [3].
P-type ATPases are a large family of membrane proteins relevant for maintaining cellular homeostasis and generating appropriate electrochemical gradients for cell survival. These enzymes use the energy released by ATP hydrolysis to catalyze the transport of cations across the cell membrane [3, 4, 5, 6]. In fact, P-type ATPases are expressed during mycobacterial infection as a response to the toxicity produced by high levels of metals in human macrophages [7, 8, 9]. There are reports of diminished vacuolar concentration of Ca2+ (1.8 1.3 mM) and K+ (19.516.9 mM) in the early phagosome (first hour after phagocytosis) as compared with extracellular bacteria [7, 10]. However, the concentrations of Ca2+ (7.1 3.3mM) and K+ (51.0 28.6mM) as well as of other metals such as copper, zinc, and iron are replenished or increased 24 h post-infection [7, 10]. Therefore, P-type ATPases, among other systems, play a critical role in maintain mycobacterial metal homeostasis during infection [7, 8, 9, 11, 12, 13, 14].
P-type ATPases are classified into five subfamilies (P1–P5), based on ionic specificity and structural characteristics [4, 6]: P1A-type bacterial potassium transporters; P1B-type heavy metal pumps; P2-type alkaline/alkaline earth metal transporters; P3A-type H+ pumps; P3B-type bacterial Mg2+ pumps; P4-type putative lipid flippases; and the uncharacterized P5-type ATPase pumps [4, 5, 6, 15]. Bioinformatic studies identified 12 P-type ATPases in the Mtb genome, namely: seven P1B-type, four P2-type, and one P1A-type ATPases [16, 17, 18]. Regarding the functional characterization of these Mtb plasma membrane pumps, CtpA is a Cu+ transporter [19], CtpC transports Mn2+ and/or Zn2+ across the plasma membrane [8, 13], CtpJ and CtpD are Fe2+, Co2+, and Ni2+ transporters [12, 14], CtpV is a Cu+ pump [9], and CtpG is a Cd2+ transporter [20]. However, the ion specificity of P2-type ATPases and/or their possible roles in mycobacteria are unknown.
Unlike eukaryotic cells, there is not a wide variety of calcium-mediated processes in bacteria [21]. However, critical physiological processes such as cellular growth, motility, quorum sensing, sporulation, and the development of different bacterial structures are regulated by the cytosolic Ca2+ concentration in bacteria [21]. All forms of life, even mycobacteria, developed mechanisms such as passive and active transporters, ion channels, and non-protein channels to regulate calcium homeostasis [21], including Ca2+-ATPases that mediate calcium homeostasis [22, 23].
Mtb CtpF is the mycobacterial P2-type ATPase most closely related to the sarco/endoplasmic reticulum calcium ATPase (SERCA1a) from eukaryotes [16]. The expression of the ctpF gene is regulated by the global mycobacterial dormancy regulator DosR [24, 25, 26, 27, 28, 29, 30, 31]. Interestingly, ctpF is activated under conditions similar to the phagosome environment and during infection [28, 32]. Specifically, ctpF responds when Mtb cells are treated with toxic substances, such as isoxyl, tetrahydrolipstatin [33], reactive nitrogen species (RNS), reactive oxygen species (ROS) [28, 29, 30, 34, 35], and under hypoxia [27, 29, 36, 37, 38]. This transcriptional behavior suggests that CtpF could be part of the strategies of the tubercle bacillus uses to face the environmental conditions encountered during infection [39]. However, the actual role of CtpF in mycobacterial ion homeostasis and its biology remains unknown. Which is the cation transported by CtpF? Are there any phenotypic consequences of ctpF overexpression and deletion in mycobacteria?
In this work, we assessed the role of CtpF in Ca2+ pumping from mycobacterial cells. Initially, the ATPase activity in the plasma membrane together with the calcium accumulation in whole cells and plasma membrane vesicles from recombinant M. smegmatis overexpressing Mtb CtpF and Mtb cells defective in ctpF (MtbΔctpF) confirmed that this transporter is associated with Ca2+ pumping from mycobacterial cells. In addition, MtbΔctpF cells were more susceptible to oxidizing agents, suggesting a link between Ca2+ transport and the mycobacterial response to oxidative stress.
2. Materials and methods
2.1. Bacterial strains and growth conditions
The bacterial strains, plasmids, and primers used in this study are listed in Tables 1 and 2. Mtb strains were grown in Middlebrook 7H9 broth supplemented with oleic acid-albumin-dextrose-catalase (OADC) (50 μg/mL oleic acid, 0.5 % Bovine albumin Fraction V, 0.2 % dextrose and 0.004 % catalase) and 0.5 % glycerol at 37 °C with gentle agitation (80 rpm) until OD600 = 0.5–0.8, or on 7H10 and 7H11 agar plates supplemented with OADC and glycerol. For the experiments of bacterial tolerance to cations, the mycobacteria were grown in Sauton's medium (pH = 7.4) supplemented with 0.05 % Tween 80 and 0.2 % glucose at 37 °C and 80 rpm. Escherichia coli DH5α and TOP10, used for plasmid propagation, were cultured at 37 °C in LB broth with agitation (180 rpm) or on LB agar plates. When required, 7H9, 7H10, and 7H11 were supplemented with 20 μg/mL kanamycin (Kan), 100 μg/mL hygromycin (Hyg), while LB was supplemented with 100 μg/mL ampicillin (Amp), 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG), 80 μg/mL X-gal, or 20 μg/mL kanamycin (Kan). Mtb genomic DNA was isolated as previously reported [40].
Table 1.
Bacterial strains and plasmids used in this study.
| Strains | Relevant features | Reference |
|---|---|---|
| Mycobacterium tuberculosis | ||
| H37Ra | Slow-growing attenuated strain, AmpR, ChxR, CbR | ATCC 25177 |
| H37Ra:pJV53 | Recombineering strain (with pJV53), AmpR, ChxR, CbR, KmR | This study |
| H37RaΔctpF | ΔctpF, gene replaced by a HygR cassette | This study |
| Mycobacterium smegmatis | ||
| mc2155 | Fast-growing, usually non-pathogenic Mycobacterium | ATCC 700084 |
| Escherichia coli | ||
| DH5α | recA-, endA-, Blue/white color screening with lacZΔM15 | Thermo Fisher Scientific |
| TOP10 | F– mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araA-leu)7697 galU galK rpsL endA1 nupG | Thermo Fisher Scientific |
| Plasmids | ||
| pMV261 | E. coli-Mycobacterium shuttle vector, hsp60 promoter, KmR | [57] |
| pLNA29 | Mtb Rv1997 (ctpF) cloned into pMV261, KmR | This study |
| pJV53 | Derivative of pLAM12 with Che9c 60–61 genes under control of the acetamidase promoter | Gift from Unizar [43] |
| pYUB854 | HygR cassette is flanked by the γδ-res sites and by two MCSs for directional cloning of the homologous recombination substrates | Gift from Unizar [58] |
| pLNA22 | 607 bp upstream and 520 bp downstream of Mtb Rv1997 (ctpF) in pYUB854 | This study |
Table 2.
List of oligonucleotides used in this study.
| Primer | Sequence (5′-3′) |
|---|---|
| F-RT Dir | CAGTGATCTTCGGTGTGGTG |
| F-RT Rev | GATTGAGCGTGAACGAGTCA |
| pMVComp Up | CAGCGAGGACAACTTGAGC |
| pMVComp Down | CGACTGCCAGGCATCAAATA |
| Fcm2 Dir | TTTTGGGATCCATTGTCGGCGTCAGTGTCTG |
| Fpmv His Rev | TTTTTAAGCTTCAATGATGATGATGATGATGTGGCGGTTGCGCCCGTA |
| pJV53dir | GTCAGTCACCAACCCTCCAC |
| pJV53rev | GAATCCTGCTTGGTGACAGC |
| ctpF_interno_dir | CTATGCACCCGACGTCCT |
| cpF_interno_rev | GAACCTGGTATCACGTTTTCG |
| Comp_Up-ctpF | TCGTCGAACACTCGTACCTG |
| Comp_Down_ctpF | CGTCCGCAACCTAGTTGAAT |
| primerpYUB854 | GTGGCTCCCTCACTTTCTGG |
| Hyg_dir_out | ACTTCGAGGTGTTCGAGGAG |
| Adir2013 | TTTTCTCGAGCGGATGGCAAGACC |
| Arev2013 | TTTTGCTAGCGCGCGTTACCACC |
| Bdir2013 | TTTTTCTAGATATCGGGGTGTGGGTGC |
| Brev2013 | TTTTTCATGATACCACCAGCACGATCCAG |
2.2. Bioinformatic analysis
The amino acid sequence of CtpF was pairwise aligned with 12 P2-type Ca2+-ATPase sequences retrieved from UniProt and the alignments were visualized using Jalview 2.10.5 software [41].
2.3. Mtb ctpF gene cloning and expression in M. smegmatis
The ctpF gene (Rv1997) was amplified by PCR from the template genomic DNA of Mtb H37Ra using the primer pair Fcm2Dir/FpmvHis-Rev (Table 2) and the Phusion DNA Polymerase (Thermo Scientific). The reverse primer added a His6-tag to the C-term of the protein. The amplimer, flanked by the BamHI and HindIII restriction sites, was cloned into the shuttle vector pMV261 to obtain the pLNA29 plasmid, whose integrity was confirmed by PCR, restriction mapping, and DNA sequencing. M. smegmatis mc2155 cells were transformed with the pLNA29 plasmid and the transformant colonies were verified by colony PCR. Recombinant colonies were grown in LB-Kan at 37 °C with agitation at 180 rpm until an OD600 = 0.5–0.6. Protein expression was induced by heat shock at 45 °C for 1 h [42]. Protein extracts were analyzed in 10% polyacrylamide gels (SDS-PAGE) and immunostaining dot-blots using rabbit anti–His polyclonal primary antibody and goat anti–rabbit secondary antibody HRP-conjugate (Thermo Scientific, USA).
2.4. Construction of the ctpF-defective Mtb strain
The deletion of the ctpF gene in Mtb was performed using the Che9c recombineering system [43]. Briefly, the allelic exchange substrate (AES) was generated by separately cloning 500 bp of the upstream and downstream sequences of the ctpF gene into the pYUB854 vector flanking a hygromycin-resistance cassette. The upstream (section A) and downstream (section B) regions of the ctpF gene were amplified by PCR and separately cloned into the pGEM®-T Easy vector (Promega). The A and B sections were subcloned into pYUB854 to generate the pLNA22 plasmid, from which the AES was released by restriction enzymes digestion. Simultaneously, the recombineering strain (Mtb transformed with the pJV53 plasmid) was cultured in 7H9 supplemented with 0.2 % succinate, 0.05 % Tween 80 and Kan, until an OD600 = 0.5–0.8. Cells were then supplemented with 0.2 M glycine and induced with 0.2% acetamide. The Recombineering cells were electroporated with 100 ng AES and plated on 7H11-OADC-Kan-Hyg [43]. Mtb colonies defective in ctpF (MtbΔctpF) were screened by colony PCR (primers listed in Table 2). Genomic DNA was isolated from the selected Mtb mutant to confirm the targeted gene replacement by PCR, using primers matching within the HygR cassette and flanking the sections A and/or B of the AES (primers listed in Table 2). Finally, the PCR products were sequenced to confirm the integrity of the targeted gene replacement.
2.5. Preparation of plasma membrane vesicles
Cultures of mycobacterial cells (5 L) were grown until OD600 = 0.5–0.8. The entire procedure was performed at 4 °C. Cells were harvested by centrifugation and washed twice with buffer A (10 mM MOPS, 0.08 g/mL sucrose, pH = 7.4), resuspended in lysis buffer (10 mM MOPS, 1 mM EDTA, 0.3 mM PMFS, pH = 7.4), and mechanically lysed with a Mini Bead Beater (Biospec) by eight 1-minute cycles. Cellular debris were removed by centrifugation at 25,000 x g for 30 min in a Megafuge 16R Centrifuge (Thermo Scientific). Supernatants (membrane and cytoplasmic fractions) were isolated by centrifugation at 100,000 x g for 90 min in a Sorvall WX Floor Ultra Centrifuge (Thermo Scientific). The remaining supernatant was discarded and the pellet containing the membrane fraction was resuspended in buffer A [44]. The protein concentration was assessed with the Bradford–Zor–Selinger [45] or the BCA methods using the Pierce BCA Protein Assay Kit (Thermo scientific). The protein extracts were finally adjusted to 1 mg/mL, aliquoted, and stored at -20 °C until use.
2.6. Metal accumulation assays
Cultures of mycobacterial cells were grown until OD600 = 0.5–0.8. Then, the cells were harvested and washed three times with washing buffer (10 mM MOPS, 140 mM choline chloride, 0.5 mM DTT, 250 mM sucrose). The cell pellet was resuspended in 5 mL in washing buffer and separately supplemented with cations using the following final concentration of salts: 10 mM CaCl2, 240 mM NaCl or 240 mM NaCl/40 mM KCl, followed by incubation at 37 °C for 1 h with agitation. Subsequently, cells were harvested and washed twice with washing buffer. Pellets were dried at 37 °C to constant weight and mineralized with 500 μL of HNO3 (trace metal grade) for 1 h at 80 °C, followed by overnight incubation at 20 °C. Sample digestions were stopped by adding 30% H2O2 and were completed with 2% high purity HNO3 in H2O (18 MOhm*cm). The metal content of the samples was measured by flame absorption spectroscopy using a 300 Atomic Absorption Spectrometer (Perkin Elmer, USA). The calcium accumulation was normalized against the amount of calcium measured in the WT cells (dry mass) [9, 12, 13].
To assess the calcium accumulation inside membrane vesicles, 10 μg of protein (membrane vesicles) were mixed with reaction buffer (25 mM MOPS, 250 mM sucrose, 3 mM MgSO4, 150 mM KCl, 0.05 % Brij-58, 3 mM Na2ATP) and incubated at 37 °C for 1 min. Reactions were initiated by adding 100 μM Ca2+, incubated at 37 °C for 30 min, stopped by filtration through 0.22 μM pore size filters (Millipore, USA), and the filters were washed two times with washing buffer (25 mM MOPS, 250 mM sucrose, 3 mM MgSO4, 150 mM KCl, 0.05 % Brij-58, 1 mM EGTA). The filters were then mineralized as above. The metal content of the samples was measured by flame absorption spectroscopy using a contrAA 700 Jena Analytik Atomic Absorption Spectrometer.
2.7. ATPase activity assays
The ATPase activity of the plasma membrane vesicles was measured according to the Fiske-Subbarow method with modifications, as previously described [19, 20, 46]. Enzymatic reactions (final volume 50 μL) were performed in 96-well plates using 10 μg of protein in reaction buffer (3 mM MgCl2, 10 mM MOPS, pH = 7.4) and supplemented with 0.02 % Brij-58, 0.29 mM Ca2+ and 0.25 mM EGTA (final concentrations) to control the amount of free calcium. Maxchelator software was used to calculate the free Ca2+ [47]. The enzymatic reactions were initiated by adding 3 mM Na2ATP and incubating at 37 °C for 30 min. The reactions were stopped by adding 100 μL of stopping solution (3 % ascorbic acid, 0.5 % ammonium molybdate, 3 % SDS and 2 M HCl). Subsequently, the samples were kept 10 min at 4 °C and then 150 μL of stabilizing solution (3.5 % bismuth citrate, 3.5 % sodium citrate, 2 M HCl) were added. Afterward, the samples were incubated at 37 °C for 10 min. Finally, the Pi released was quantified by measuring the OD690. The ATPase activity is reported as Pi nmol produced by mg protein by min of reaction (nmol Pi. mg−1. min−1) from three independent experiments [19, 20, 46].
2.8. Mycobacterial tolerance to metal cations
The culture was harvested and washed three times with Sauton's medium (pH = 7.4) supplemented with 0.05 % Tween 80 and 0.2 % glucose. The cell pellet was resuspended in Sauton's medium and diluted until OD600=0.05–0.06. Subsequently, 100 μL of bacterial suspension were separately mixed in 96-well plates with 100 μL of serial dilutions of cations in a range of previously determined concentrations: Ca2+ (0.4 mM - 9.5mM), Na+ (10 mM–150 mM) and K+ (5 mM–150 mM).
Cultures were incubated at 37 °C for 21 days at 80 rpm and the final OD600 of cultures was measured in an iMARKTM Microplate Reader (Bio-Rad, CA, USA). Cells grown in the same medium without cation or supplemented only with 10 μg/mL isoniazid were considered controls for 100 % and 0 % growth, respectively [19, 20]. The IC50 was determined using GraphPad Prism 8.0 software using a nonlinear regression with log (inhibitor) vs. normalized response -Variable slope. Each experiment was assessed in triplicate from three biological replicates.
2.9. Oxidative and nitrosative stress assays
Cultures of mycobacterial cells were harvested and washed three times with 7H9 supplemented with oleic acid-albumin-dextrose (OAD) (50 μg/mL oleic acid, 0.5 % Bovine albumin Fraction V and 0.2 % dextrose) and 0.05 % Tween 80. The cell pellet was resuspended in 7H9-OAD and diluted until an OD600 = 0.05–0.06. Subsequently, 100 μL of bacterial suspension were separately mixed in 96-well plates with 100 μL of serial dilutions of redox agents in a range of previously determined concentrations: H2O2 (0.5 mM–25 mM) and sodium nitroprusside (SNP) (0.01 mM–1 mM) [29, 34, 48]. Cultures were incubated at 37 °C for 8 days at 80 rpm and the final OD600 of cultures were measured in an iMARKTM Microplate Reader (Bio-Rad, CA, USA). Cells grown in the same medium without an oxidant agent or supplemented only with 10 μg/mL isoniazid were considered controls for 100 % and 0 % growth, respectively. The IC50 was determined in GraphPad Prism 8.0 software using nonlinear regression with log (inhibitor) vs. normalized response -Variable slope. The results are representative of three independent experiments.
3. Results
3.1. Mtb ctpF encodes a putative Ca2+ P-type ATPase
Among the 12 ORFs that encode P-type ATPases in the Mtb genome, four of them have been classified as P2-type ATPases: CtpE, CtpF, CtpI, and CtpH [16]. This subclass of membrane transporters includes calcium transport-associated enzymes such as SERCA, PMCA1, SPCA and LMCA1, together with Na+/K+-ATPases [49]. SERCA is the best structurally characterized P2-type ATPase, and multiple conformations comprising its entire catalytic cycle have been resolved and reported in the Protein Data Bank. In addition, SERCA is highly conserved in higher eukaryotes and displays high homology with some prokaryotic organisms. In contrast, no X-ray structures of bacterial Ca2+ P-type ATPases have been resolved. There are only biochemical characterizations of some bacterial Ca2+ P-type ATPases from Listeria monocytogenes and Streptococcus pneumoniae [22, 50].
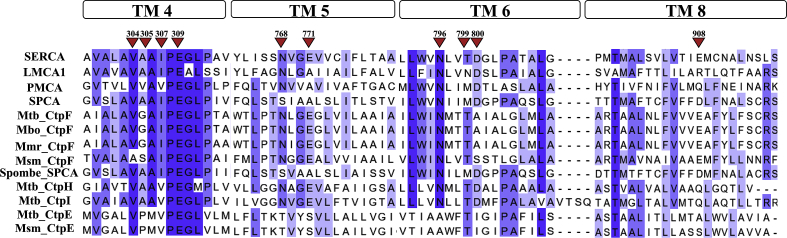
Members of the P2-type ATPases group share the characteristic PEGL-motif and the conserved amino acids of the ion-binding pocket [51]. As shown in Fig. 1, CtpF is closely related to SERCA by sharing 8 of the 10 residues involved in calcium binding in SERCA [52]. The calcium coordination sites in SERCA are named Site I (Asn768, Glu771, Thr799, Asp800, Glu908) and Site II (Val304, Ala305, Ile307, Glu309, Asn796, Asp800) [52]. We observed that CtpF displays all of these residues, except for Ala305 that is substituted by a Gly residue and Asp800 that is substituted by an Ala residue (Fig. 1). Thus, even when the primary structures of CtpF (905 aa; 95 kDa) and SERCA (994 aa; 110 kDa) display just a 33% overall identity, they share an 80% identity with respect to the residues involved in Ca2+ coordination.
Fig. 1.
Mtb CtpF possesses the characteristic Ca2+ binding residues of P-type ATPases. Multiple sequence alignment of 12 P2-type ATPases in MEGA X using Muscle. All sequences were retrieved from UniProt. Jalview 2.10.5 was used to visualize the final result. The arrows show the 10 key calcium-binding residues of SERCA, 8 of which are conserved in CtpF. Blocks in top represent the transmembrane segments (TM) were the cation binding amino acids are located.
3.2. CtpF prevents calcium accumulation in mycobacterial cells
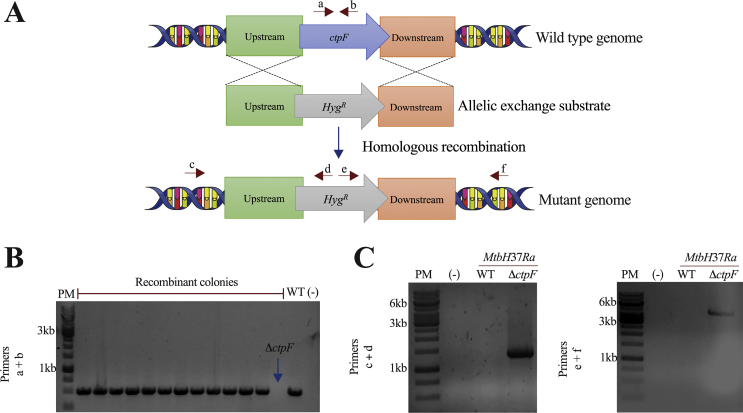
In order to determine if CtpF is involved in calcium transport across the mycobacterial cell membrane, we evaluated calcium accumulation in recombinant obtained from M. smegmatis cells expressing CtpF, Mtb WT and MtbΔctpF cells. The latest were obtained by homologous recombination using the Che9c system (Fig. 2A) [43]. The allelic exchange replacement of the ctpF locus in the MtbΔctpF mutant cells was confirmed by PCR (Fig. 2B and C). Nucleotide sequencing of the amplimers showed that the Hyg cassette indeed inserted into the ctpF gene, by showing the AES insertion into the desired site of the Mtb genome and the presence of the γδ resolvase sites, allowing the further removal of the Hygromycin resistance cassette to eliminate the antibiotic resistance gene [43].
Fig. 2.
Targeted allelic exchange of the Mtb ctpF locus. A) Schematic representation of the homologous recombination process to generate a ctpF knockout mutant of Mtb. B) Thirteen colonies were examined by PCR using primers a and b (508 pb for the WT locus and no product for the mutant). The recombinant strain showing the absence of the ctpF gene was selected and named MtbΔctpF. DNA from Mtb WT was used as control. C) A PCR analysis was performed using different combinations of primers c and d or e and f to confirm the homologous recombination in the target locus; no PCR product is expected from WT cells. Primers d and e are located within the HygR cassette and only match in the chromosome of mutant strain.
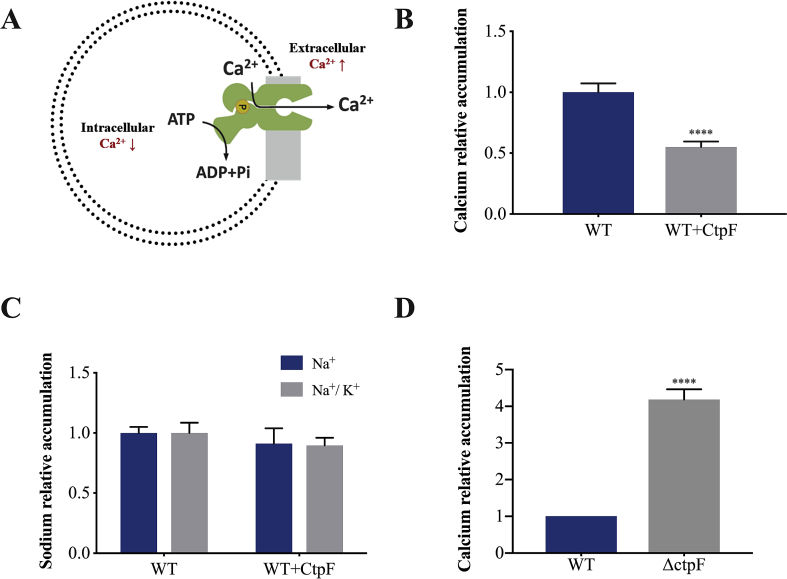
When exposing the M. smegmatis cells expressing CtpF to a high calcium concentration in the extracellular medium (10 mM) during 1 h, the recombinant cells accumulate 2-fold less Ca2+ than the control cells (WT transformed with empty pMV261). This suggests that CtpF may be involved in the active Ca2+ transport from mycobacterial cells against the concentration gradient (Figs. 3A and 3B). To confirm that it was an ion-specific transport, this experiment was also performed using 240 mM sodium and 240 mM/40 mM sodium/potassium treatment. No significant differences were observed between recombinant and control cells under those conditions (Fig. 3C). To further confirm that CtpF is associated with calcium efflux from mycobacterial cells, we determined the calcium accumulation in the MtbΔctpF strain. In agreement, MtbΔctpF cells accumulated 4-fold more Ca2+ than the Mtb WT cells (Fig. 3D). These results are consistent with the reduced calcium accumulation observed for the recombinant strain and supports the hypothesis that CtpF is a calcium efflux pump (Fig. 3A).
Fig. 3.
Calcium accumulation in mycobacterial cells. A) Hypothesized function of CtpF in the mycobacterial plasma membrane. In the presence of a high extracellular calcium concentration, CtpF transports calcium against its concentration gradient. B) Calcium accumulation inside M. smegmatis WT and the recombinant cells expressing CtpF. The amount of calcium accumulated was internally normalized against the amount of calcium measured in the dry mass of pellet from the WT strain. C) Sodium accumulation in the absence and presence of potassium in the WT and recombinant strains normalized with respect to the metal accumulation in the WT strain. D) Calcium accumulation in Mtb cells. The amount of calcium accumulated was internally normalized against the amount of calcium measured in the dry mass of pellet from the WT strain. The data shown are representative of three independent experiments. Unpaired two-tailed t test, ****P < 0.0001.
3.3. CtpF promotes calcium accumulation in right-side-out mycobacterial plasma membrane vesicles
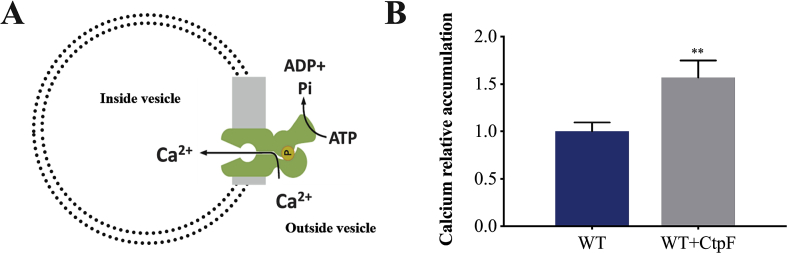
To further confirm that CtpF transports calcium from inside mycobacterial cells against the concentration gradient, we measured calcium accumulation in an inverted membrane vesicle model supplemented with Brij 58 to obtain an "inside-out" configuration (Fig. 4A) [44]. In this experimental model, the cytoplasmic domains of CtpF are exposed to the reaction medium, the direction of transport is inverted, and calcium accumulation depends on the availability of ATP and Ca2+in the reaction milieu. As shown in Fig. 4B, vesicles obtained from recombinant M. smegmatis cells accumulated more Ca2+ than vesicles obtained from WT cells, confirming that CtpF transports the metal from the cytoplasm.
Fig. 4.
Calcium accumulation in membrane vesicles obtained from M smegmatis cells. A) Model of inverted membrane vesicles where the cytoplasmic domains of CtpF are exposed to the reaction medium. B) Calcium accumulation in WT and recombinant membrane vesicles from cells expressing ctpF. All normalized against the calcium accumulation in membrane vesicles of the WT strain. Data corresponds to mean ± SEM from three independent replicates. Unpaired two-tailed t test, **P < 0.01.
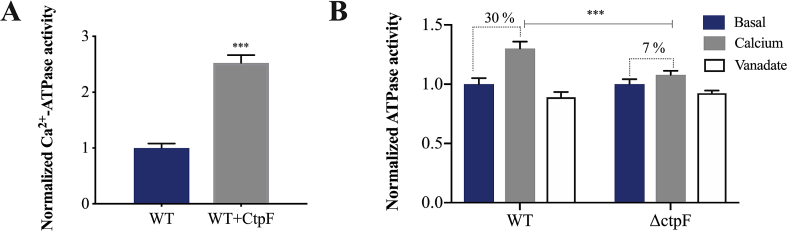
3.4. Ca2+ ions stimulate the CtpF ATPase activity
We assessed the Ca2+-dependent ATPase activity on plasma membrane vesicles obtained from mycobacterial cells. The enzyme reactions were supplemented with ATP as energy source, Mg2+ as cofactor, and Brij-58. Then, the ATPase activity was measured by quantifying the Pi released from ATP hydrolysis [44, 46]. As expected, the Ca2+-ATPase activity was higher in vesicles from M. smegmatis cells expressing CtpF (2.5-fold) than in vesicles obtained from M. smegmatis WT cells (Fig. 5A). In agreement, Ca2+ ATPase activity increased only 7 % in the MtbΔctpF plasma membrane vesicles, when the increment was 30 % in vesicles obtained from the Mtb WT cells, compared to the basal ATPase activity (Fig. 5B). This decreased Ca2+-dependent ATPase activity in vesicles from MtbΔctpF cells is in agreement with the proposed role of CtpF, as Ca2+-efflux pump. Importantly, since the ATPase activity was measured on crude membrane extracts, part of the Ca2+-stimulated ATPase activity in vesicles from MtbΔctpF cells should be attributed to other Ca2+ATPases present in the mycobacterial plasma membrane. Corroborating CtpF is a Ca2+ P-type ATPase, its activity was susceptible to vanadate (Fig. 5B), a known inhibitor of this kind of transporters [44, 46, 53].
Fig. 5.
Ca2+ P-type ATPase mediated by CtpF. A) Ca2+-dependent ATPase activity of membrane vesicles of recombinant M. smegmatis normalized against the control strain (M. smegmatis WT). B) Ca2+-ATPase activity of membrane vesicles of Mtb WT and MtbΔctpF against the basal ATPase activity. The dotted lines represent the percentage of the ATPase activity stimulated by Ca2+. Values correspond to the mean ± SEM from three independent replicates. Unpaired two-tailed t test, ***P < 0.001.
3.5. CtpF confers Ca2+ tolerance to mycobacterial cells
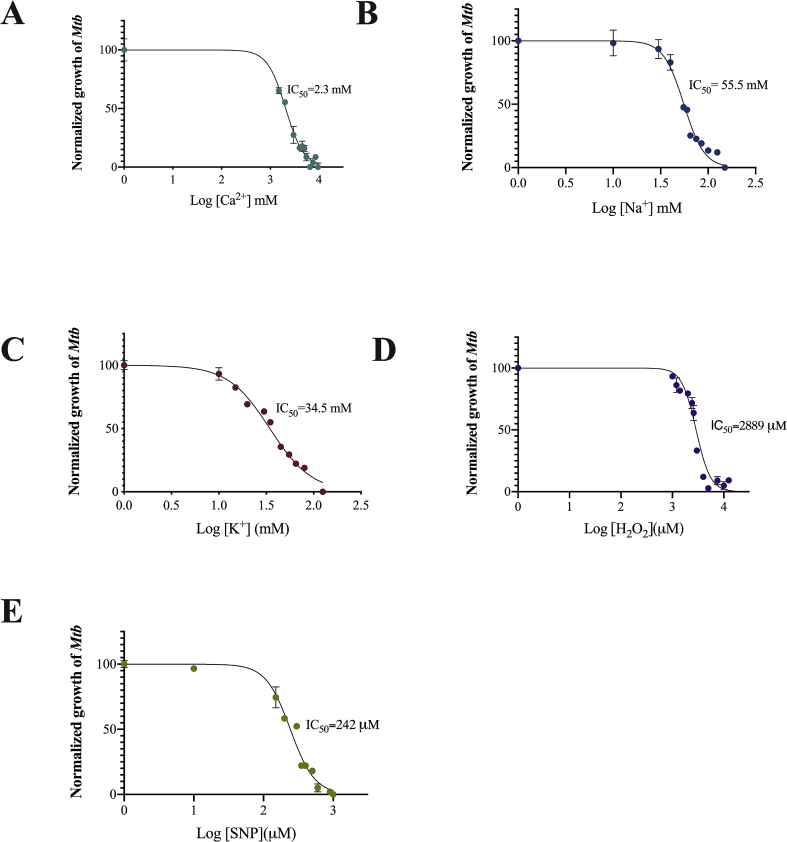
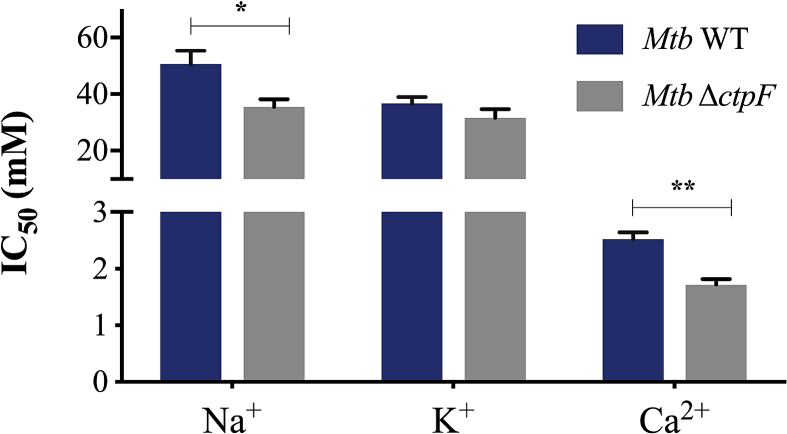
Since Mtb activates P-type ATPases to face high concentrations of metals inside macrophages [7, 8, 9], it is possible that CtpF may contribute to restoring intracellular calcium levels in Mtb. Therefore, we assessed the tolerance of Mtb cells to different concentrations of Ca2+, K+, and Na+ (Fig. 6). Assuming that CtpF is a potential calcium transporter, MtbΔctpF should be sensitive to high concentrations of calcium. As observed in Fig. 7, MtbΔctpF cells were more susceptible to Ca2+ than WT. Specifically, the IC50 value of calcium in MtbΔctpF cells (1.7 mM) was significantly lower than in WT cells (2.5 mM). Even though the tolerance of MtbΔctpF to Na+ was significantly lower than WT, no difference in the tolerance to of K+ was observed (Fig. 7). These results reinforce the idea that CtpF contributes to maintaining the physiological level of calcium in the mycobacterial cytosol.
Fig. 6.
Growth of Mtb cells in presence of cations and oxidizing agents. Mtb strains were grown in Sauton's media supplemented with varying concentrations of A) CaCl2. B) NaCl. C) KCl. 7H9-OAD media supplemented with varying concentrations of D) H2O2. E) SNP. The OD600 of mycobacteria growing in absence of cations or oxidizing agents was considered as positive control (100% of cell growth).
Fig. 7.
Tolerance of Mtb cells to metal cations. Mycobacterial cells were grown in Sauton media supplemented with varying concentrations of CaCl2, KCl, and NaCl. OD600 of cultures supplemented without cations were considered as 100% of cell growth. Values represent the IC50. Data are mean ± SEM from three independent experiments. Unpaired two-tailed t test, *P < 0.05, **P < 0.01.
3.6. CtpF is associated with the oxidative stress response in mycobacterial cells
Being part of the DosRS regulon [24, 25, 26, 27, 28, 29, 30, 31], we suspected a possible association between the transcriptional response of ctpF and oxidative/nitrosative stress. Testing this, we evaluated the sensitivity of MtbΔctpF and Mtb WT to ROS/RNS stress conditions (Figs. 6 and 8). As observed in Fig. 8, MtbΔctpF cells were more sensitive to oxidative and nitrosative stresses compared to Mtb WT. With IC50 values of 1.6 mM H2O2 and 85 μM SNP for MtbΔctpF cells and 2.9 mM H2O2 and 214 μM SNP for Mtb WT, confirming that MtbΔctpF cells displayed hyper-susceptibility to ROS/RNS stresses compared to the WT strain.
Fig. 8.
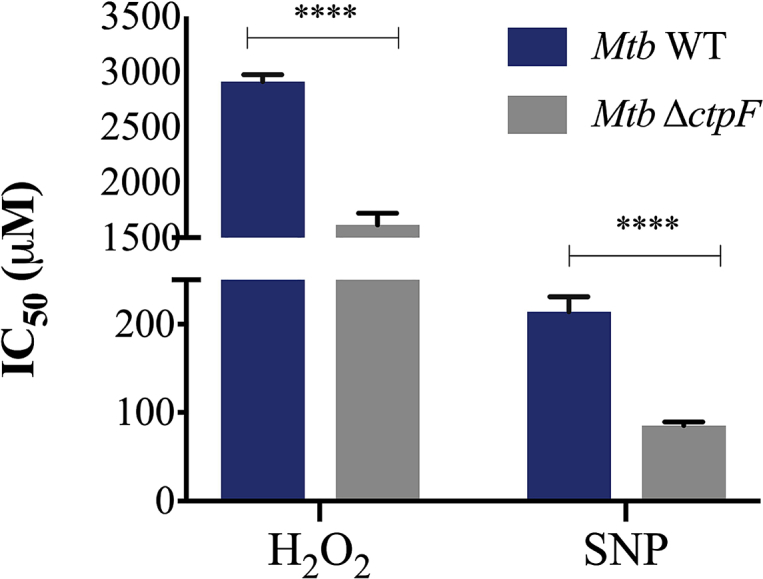
Response of Mtb WT and MtbΔctpF to oxidative and nitrosative stresses. Bacteria were grown in 7H9-OAD media supplemented with varying concentrations of H2O2 and SNP. OD600 of cultures in absence of oxidant agent are considered the 100%. Values represent the IC50. Data are mean ± SEM from three independent experiments. Unpaired two-tailed t test, ****P < 0.0001.
4. Discussion
Calcium is a pivotal messenger for different physiological processes and signaling cascades in bacteria [50, 54]. Calcium is directly involved in membrane transport mechanisms, chemotaxis, cell division, and differentiation processes [54, 55]. The intracellular calcium content in bacteria increases when cells are surrounded by natural environments containing high doses of this metal (millimolar calcium concentrations). However, this increase in calcium levels should be transient in order to maintain bacterial viability [50, 54]. Therefore, a calcium homeostatic system is essential for bacterial survival [50, 54]. In this sense, Ca2+ P-type ATPases may play a relevant essential role in bacterial integrity.
CtpF is the most closely related mycobacterial transporter to the well-studied calcium eukaryotic transporter SERCA. Accordingly, our bioinformatics predictions showed that both proteins share 8 of 10 amino acids from the calcium coordination sites. Since other bacterial calcium ATPases also share key calcium-binding residues with SERCA, such as LMCA1 from L. monocytogenes [22], we hypothesized that CtpF is a Ca2+-transporting P-type ATPase. Testing this, calcium accumulation experiments were conducted on whole mycobacterial cells and plasma membrane vesicles. M. smegmatis was used as an expression host for CtpF since this environmental species is an easier-to-handle model with similar envelope and membrane properties to Mtb [56]. The calcium accumulation experiments indicated that M. smegmatis plasma membrane with MtbCtpF embedded responded to increased extracellular calcium concentration. Even though, this could be attributable not only to P-type ATPases but also to the activity of different metal transport systems [21], we consider that this reduced calcium accumulation in M. smegmatis recombinant cells to the presence of CtpF in the membrane, which is the relevant difference between the recombinant and control cells. To further discard that CtpF is a sodium/potassium P2-type ATPase [46], we tested sodium accumulation. The results showed that there is no significant difference in the accumulation of sodium between recombinant and control cells. These results suggest a calcium efflux activity mediated by CtpF.
Further evidence for CtpF-mediated calcium transport to the extracellular medium was provided by experiments using a membrane vesicle model, in which, hypothetically, the direction of calcium transport by CtpF is reversed [44]. This approach was complemented by measuring ATPase activity. As expected, calcium accumulation and Ca2+-dependent ATPase activity were higher in the membrane vesicles from the recombinant cells compared to control cells. This validates the hypothesis that the direction of transport was inverted in plasma membrane vesicles. The mutant phenotype of the MtbΔctpF corroborates that CtpF is a calcium efflux pump. This is: 1) MtbΔctpF cells have an impared capacity to restore cytoplasmic calcium levels upon extracellular Ca2+ exposure; 2) Vesicles isolated from MtbΔctpF exhibited a reduced Ca2+-dependent ATPase activity and 3) MtbΔctpF cells are more susceptible to Ca2+ than WT cells. Therefore, CtpF plays an important role in calcium homeostasis by preventing a toxic metal overload and favoring mycobacterial survival under infection conditions.
Undoubtedly, Mtb requires an efflux system to maintain calcium homeostasis when the tubercle bacillus is surviving in highly enriched calcium environments, such as lungs and mucous membranes [50]. The deletion of P-type ATPases in mycobacteria is known to cause unbalanced cation transport across the plasma membrane and impaired capacity to respond to toxic substances [8, 9, 12, 13, 14]. It is likely that different concerted cellular events contribute to maintaining the optimal levels of intracellular calcium, including: 1) calcium influx; 2) an increase in the intracellular calcium concentration; 3) calcium binding of target proteins, and 4) restoration of the calcium concentration by inducing a metal efflux mechanisms, in which CtpF could be relevant.
There are reports that intracellular calcium accumulation in S. pneumoniae activates molecular systems in response to oxidative stress; indeed, S. pneumonia defective in Ca2+-ATPase is more sensitive to oxidative stress compared with the WT strain [50]. In agreement, our data showing the hypersensitivity to oxidizing agents of the MtbΔctpF strain suggest a correlation between calcium pumping and oxidative stress response in mycobacteria. This is in agreement with ctpF expression being regulated by the dormancy regulator DosR in response to low levels of O2 and nitric oxide exposure [26, 27, 29, 34, 38]. We speculate that CtpF is activated in response redox stress, a characteristic stress condition faced by pathogens during infection. This implies a critical function of CtpF in Mtb; however, experiments evaluating the importance of CtpF during infection are necessary to further confirm this hypothesis.
5. Conclusions
CtpF resembles SERCA, the well-studied P2-type Ca2+-ATPase in higher eukaryotes. Our data show that CtpF is in fact a bacterial Ca2+-ATPase. The expression of CtpF in M. smegmatis allows recombinant cells to withstand a transient increase in calcium concentration. Accordingly, MtbΔctpF cells exhibit higher calcium accumulation and increased susceptibility to the cation. The increased susceptibility of the MtbΔctpF strain to oxidative stress, suggest a link of the efflux pump function of CtpF with more complex cellular processes, as the response to toxic substances encountered during infection, or bacterial signaling in other to respond to the arsenal of the host cell. In conclusion, our experiments indicate that CtpF transports calcium from mycobacterial cells to the extracellular environment against the concentration gradient.
Declarations
Author contribution statement
Milena Maya-Hoyos, Cristian Rosales: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Lorena Novoa-Aponte: Conceived and designed the experiments; Wrote the paper.
Eliana Castillo: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Carlos Yesid Soto: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Colciencias Grant 110171250419 and the División de Investigación Bogotá (DIB)-Universidad Nacional de Colombia, grant 41646. MMH and CR are fellows of Colciencias-Colfuturo Doctorado Nacional 647 (2015–2019) and the Vicerrectoría de Investigación/Universidad Nacional de Colombia, respectively. LNA was fellow of Colciencias-Colfuturo Doctorado Nacional 567.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank José Ainsa from Universidad de Zaragoza for providing the pJV53 and pYUB854 plasmids.
References
- 1.World Health Organization . 2018. Global Tuberculosis Report 2018. [Google Scholar]
- 2.Andersen P., Scriba T.J. Moving tuberculosis vaccines from theory to practice. Nat. Rev. Immunol. 2019;19:550–562. doi: 10.1038/s41577-019-0174-z. [DOI] [PubMed] [Google Scholar]
- 3.Yatime L., Buch-Pedersen M.J., Musgaard M., Morth J.P., Winther A.M.L., Pedersen B.P., Olesen C., Andersen J.P., Vilsen B., Schiøtt B., Palmgren M.G., Møller J.V., Nissen P., Fedosova N. P-type ATPases as drug targets: tools for medicine and science. Biochim. Biophys. Acta Bioenerg. 2009;1787:207–220. doi: 10.1016/j.bbabio.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Morth J.P., Pedersen B.P., Buch-Pedersen M.J., Andersen J.P., Vilsen B., Palmgren M.G., Nissen P. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 2011;12:60–70. doi: 10.1038/nrm3031. [DOI] [PubMed] [Google Scholar]
- 5.Bublitz M., Poulsen H., Morth J.P., Nissen P. In and out of the cation pumps: P-Type ATPase structure revisited. Curr. Opin. Struct. Biol. 2010;20:431–439. doi: 10.1016/j.sbi.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Palmgren M.G., Nissen P. P-type ATPases. Annu. Rev. Biophys. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 7.Soldati T., Neyrolles O. Mycobacteria and the intraphagosomal environment: take it with a pinch of salt(s)! Traffic. 2012;13:1042–1052. doi: 10.1111/j.1600-0854.2012.01358.x. [DOI] [PubMed] [Google Scholar]
- 8.Botella H., Peyron P., Levillain F., Poincloux R., Poquet Y., Brandli I., Wang C., Tailleux L., Tilleul S., Charrire G.M., Waddell S.J., Foti M., Lugo-Villarino G., Gao Q., Maridonneau-Parini I., Butcher P.D., Castagnoli P.R., Gicquel B., De Chastellier C., Neyrolles O. Mycobacterial P 1-Type ATPases mediate resistance to Zinc poisoning in human macrophages. Cell Host Microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward S.K., Abomoelak B., Hoye E.A., Steinberg H., Talaat A.M. CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2010;77(5):1096–1110. doi: 10.1111/j.1365-2958.2010.07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner D., Maser J., Lai B., Cai Z., Barry C.E., Honer zu Bentrup K., Russell D.G., Bermudez L.E. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J. Immunol. 2005;174(3):1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 11.Arguello J.M., Gonzalez-Guerrero M., Raimunda D. Bacterial transition metal P(1B)-ATPases: transport mechanism and roles in virulence. Biochemistry. 2011;50:9940–9949. doi: 10.1021/bi201418k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raimunda D., Long J.E., Padilla-Benavides T., Sassetti C.M., Argüello J.M. Differential roles for the Co2+/Ni2+ transporting ATPases, CtpD and CtpJ, in Mycobacterium tuberculosis virulence. Mol. Microbiol. 2014;91:185–197. doi: 10.1111/mmi.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padilla-Benavides T., Long J.E., Raimunda D., Sassetti C.M., Argüello J.M. A novel P1B-type Mn2+-transporting ATPase is required for secreted protein metallation in mycobacteria. J. Biol. Chem. 2013;288:11334–11347. doi: 10.1074/jbc.M112.448175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel S.J., Lewis B.E., Long J.E., Nambi S., Sassetti C.M., Stemmler T.L., Argüello J.M. Fine-tuning of substrate affinity leads to alternative roles of mycobacterium tuberculosis Fe2+-ATPases. J. Biol. Chem. 2016;291(22):11529–11539. doi: 10.1074/jbc.M116.718239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith A.T., Smith K.P., Rosenzweig A.C. Diversity of the metal-transporting P1B-type ATPases. J. Biol. Inorg. Chem. 2014;19(6):947–960. doi: 10.1007/s00775-014-1129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novoa-Aponte L., Leon-Torres A., Patino-Ruiz M., Cuesta-Bernal J., Salazar L.M., Landsman D., Marino-Ramirez L., Soto C.Y. In silico identification and characterization of the ion transport specificity for P-type ATPases in the Mycobacterium tuberculosis complex. BMC Struct. Biol. 2012;12:25. doi: 10.1186/1472-6807-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agranoff D. Metal ion transport and regulation in mycobacterium tuberculosis. Front. Biosci. 2004;9:2996–3006. doi: 10.2741/1454. [DOI] [PubMed] [Google Scholar]
- 18.Cole S.T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S.V., Eiglmeier K., Gas S., Barry C.E., Tekaia F., Badcock K., Basham D., Brown D., Chillingworth T., Connor R., Davies R., Devlin K., Feltwell T., Gentles S., Hamlin N., Holroyd S., Hornsby T., Jagels K., Krogh A., McLean J., Moule S., Murphy L., Oliver K., Osborne J., Quail M.A., Rajandream M.A., Rogers J., Rutter S., Seeger K., Skelton J., Squares R., Squares S., Sulston J.E., Taylor K., Whitehead S., Barrell B.G. Deciphering the biology of mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 19.León-Torres A., Novoa-Aponte L., Soto C.Y. CtpA, a putative Mycobacterium tuberculosis P-type ATPase, is stimulated by copper (I) in the mycobacterial plasma membrane. Biometals. 2015;28:713–724. doi: 10.1007/s10534-015-9860-x. [DOI] [PubMed] [Google Scholar]
- 20.López M., Quitian L.V., Calderón M.N., Soto C.Y. The P-type ATPase CtpG preferentially transports Cd2+across the Mycobacterium tuberculosis plasma membrane. Arch. Microbiol. 2018;200(3):483–492. doi: 10.1007/s00203-017-1465-z. [DOI] [PubMed] [Google Scholar]
- 21.Campbell A.K. first ed. John Wiley & Sons; 2015. Intracellular Calcium. [Google Scholar]
- 22.Faxén K., Andersen J.L., Gourdon P., Fedosova N., Morth J.P., Nissen P., Møller J.V. Characterization of a Listeria monocytogenes Ca2+ pump: a SERCA-type ATPase with only one Ca2+-binding site. J. Biol. Chem. 2011;286(2):1609–1617. doi: 10.1074/jbc.M110.176784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta H.K., Shrivastava S., Sharma R. A Novel Calcium Uptake Transporter of Uncharacterized P-type ATPase Family Supplies Calcium for Cell Surface Integrity in Mycobacterium Smegmatis. mBio. 2017;8(5):e01388–17. doi: 10.1128/mBio.01388-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulido P.a., Novoa-Aponte L., Villamil N., Soto C.Y. The DosR dormancy regulator of Mycobacterium tuberculosis stimulates the Na+/K+ and Ca2+ ATPase activities in plasma membrane vesicles of mycobacteria. Curr. Microbiol. 2014;69:604–610. doi: 10.1007/s00284-014-0632-6. [DOI] [PubMed] [Google Scholar]
- 25.Bacon J., James B.W., Wernisch L., Williams A., Morley K.A., Hatch G.J., Mangan J.A., Hinds J., Stoker N.G., Butcher P.D., Marsh P.D. The influence of reduced oxygen availability on pathogenicity and gene expression in Mycobacterium tuberculosis. Tuberculosis. 2004;84(3–4):205–217. doi: 10.1016/j.tube.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Kendall S.L., Movahedzadeh F., Rison S.C.G., Wernisch L., Parish T., Duncan K., Betts J.C., Stoker N.G. The Mycobacterium tuberculosis dosRS two-component system is induced by multiple stresses. Tuberculosis. 2004;84(3–4):247–255. doi: 10.1016/j.tube.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Park H.D., Guinn K.M., Harrell M.I., Liao R., Voskuil M.I., Tompa M., Schoolnik G.K., Sherman D.R. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 2003;48(3):833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnappinger D., Ehrt S., Voskuil M.I., Liu Y., Mangan J.A., Monahan I.M., Dolganov G., Efron B., Butcher P.D., Nathan C., Schoolnik G.K. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages insights into the phagosomal environment. J. Exp. Med. 2003;198(5):693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voskuil M.I., Schnappinger D., Visconti K.C., Harrell M.I., Dolganov G.M., Sherman D.R., Schoolnik G.K. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 2003;198(5):705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiloh M.U., Manzanillo P., Cox J.S. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe. 2008;3(5):323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A., Deshane J.S., Crossman D.K., Bolisetty S., Yan B.S., Kramnik I., Agarwal A., Steyn A.J.C. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J. Biol. Chem. 2008;283(26):18032–18039. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tailleux L., Waddel S.J., Pelizzola M., Mortellaro A., Withers M., Tanne A., Castagnoli P.R., Gicquel B., Stoker N.G., Butcher P.D., Foti M., Neyrolles O. Probing host pathogen cross-talk by transcriptional profiling of both Mycobacterium tuberculosis and infected human dendritic cells and macrophages. PLoS One. 2008;3(1):e1403. doi: 10.1371/journal.pone.0001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waddell S.J., Stabler R.A., Laing K., Kremer L., Reynolds R.C., Besra G.S. The use of microarray analysis to determine the gene expression profiles of Mycobacterium tuberculosis in response to anti-bacterial compounds. Tuberculosis. 2004;84(3–4):263–274. doi: 10.1016/j.tube.2003.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohno H., Zhu G., Mohan V.P., Chu D., Kohno S., Jacobs W.R., Chan J. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell Microbiol. 2003;5(9):637–648. doi: 10.1046/j.1462-5822.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- 35.Boshoff H.I.M., Myers T.G., Copp B.R., McNeil M.R., Wilson M.A., Barry C.E. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism. Novel insights into drug mechanisms of action. J. Biol. Chem. 2004;279(38):40174–40184. doi: 10.1074/jbc.M406796200. [DOI] [PubMed] [Google Scholar]
- 36.Cho S.H., Goodlett D., Franzblau S. ICAT-based Comparative Proteomic Analysis of Non-replicating Persistent Mycobacterium tuberculosis. Tuberculosis. 2006;86(6):445–460. doi: 10.1016/j.tube.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Muttucumaru D.G.N., Roberts G., Hinds J., Stabler R.A., Parish T. Gene Expression Profile of Mycobacterium tuberculosis in a Non-replicating State. Tuberculosis. 2004;84(3–4):239–246. doi: 10.1016/j.tube.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Sherman D.R., Voskuil M., Schnappinger D., Liao R., Harrell M.I., Schoolnik G.K. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding -crystallin. Proc. Natl. Acad. Sci. 2001;98(13):7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novoa-Aponte L., Soto Ospina C.Y. Mycobacterium tuberculosis p-type atpases: possible targets for drug or vaccine development. BioMed Res. Int. 2014;2014:296986. doi: 10.1155/2014/296986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somerville W., Thibert L., Schwartzman K., Behr M.A. Extraction of Mycobacterium tuberculosis DNA: a question of containment. J. Clin. Microbiol. 2005;43(6):2996–2997. doi: 10.1128/JCM.43.6.2996-2997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waterhouse A.M., Procter J.B., Martin D.M.A., Clamp M., Barton G.J. Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9):1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L., Zhong Q., Bao L., Zhang Y., Gao L., Huang B., Zhang H.-D. Rv0901 from Mycobacterium tuberculosis, a possible novel virulent gene proved through the recombinant Mycobacterium smegmatis. Jpn. J. Infect. Dis. 2009;62(1):26–31. [PubMed] [Google Scholar]
- 43.Van Kessel J.C., Hatfull G.F. Mycobacterial recombineering. Methods Mol. Biol. 2008;435:203–215. doi: 10.1007/978-1-59745-232-8_15. [DOI] [PubMed] [Google Scholar]
- 44.Santos P., Gordillo A., Osses L., Salazar L.M., Soto C.Y. Effect of Antimicrobial Peptides on ATPase Activity and Proton Pumping in Plasma Membrane Vesicles Obtained from Mycobacteria. Peptides. 2012;36(1):121–128. doi: 10.1016/j.peptides.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Zor T., Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal. Biochem. 1996;236(2):302–308. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]
- 46.Ayala-Torres C., Novoa-Aponte L., Soto C.Y. Pma1 is an alkali/alkaline earth metal cation ATPase that preferentially transports Na+ and K+ across the Mycobacterium smegmatis plasma membrane. Microbiol. Res. 2015;176:1–6. doi: 10.1016/j.micres.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Bers D.M., Patton C.W., Nuccitelli R. 2010. A Practical Guide to the Preparation of Ca2+ Buffers. [DOI] [PubMed] [Google Scholar]
- 48.Voskuil M.I., Bartek I.L., Visconti K., Schoolnik G.K. The response of Mycobacterium tuberculosis to reactive oxygen and nitrogen species. Front. Microbiol. 2011;2:105. doi: 10.3389/fmicb.2011.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Axelsen K.B., Palmgren M.G. Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol. 1998;46(1):84–101. doi: 10.1007/pl00006286. [DOI] [PubMed] [Google Scholar]
- 50.Rosch J.W., Sublett J., Gao G., Wang Y.D., Tuomanen E.I. Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol. Microbiol. 2008;70(2):435–444. doi: 10.1111/j.1365-2958.2008.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan H., Babayan V., Blyumin E., Gandhi C., Hak K., Harake D., Kumar K., Lee P., Li T.T., Liu H.Y., Lo T.C.T., Meyer C.J., Stanford S., Zamora K.S., Saier M.H. The P-Type ATPase superfamily. J. Mol. Microbiol. Biotechnol. 2010;19(1–2):5–104. doi: 10.1159/000319588. [DOI] [PubMed] [Google Scholar]
- 52.Musgaard M., Thøgersen L., Schiøtt B., Tajkhorshid E. Tracing cytoplasmic Ca 2+ ion and water access points in the Ca 2+ -ATPase. Biophys. J. 2012;102(2):268–277. doi: 10.1016/j.bpj.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clausen J.D., Bublitz M., Arnou B., Olesen C., Andersen J.P., Møller J.V., Nissen P. Crystal Structure of the Vanadate-Inhibited Ca2+-ATPase. Structure. 2016;24(4):617–623. doi: 10.1016/j.str.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 54.Domínguez D.C. Calcium Signal Transduct. 2018. Calcium signaling in prokaryotes. [Google Scholar]
- 55.Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bashiri G., Baker E.N. Production of recombinant proteins in Mycobacterium smegmatis for structural and functional studies. Protein Sci. 2015;24(1):1–10. doi: 10.1002/pro.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stover C.K., de la Cruz V.F., Fuerst T.R., Burlein J.E., Benson L.A., Bennett L.T., Bansal G.P., Young J.F., Lee M.H., Hatfull G.F. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 58.Bardarov S., Bardarov S., Pavelka M.S., Sambandamurthy V., Larsen M., Tufariello J.A., Chan J., Hatfull G., Jacobs W.R. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;10:3007–3017. doi: 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]