Figure 3.
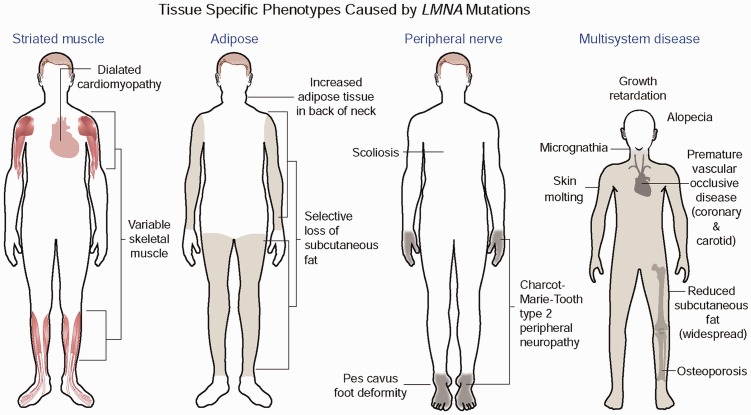
LMNA mutations cause four main disease phenotypes. Most dominant LMNA mutations cause striated muscle disease. The diagram shows the classical Emery-Dreifuss muscular dystrophy, which is characterized by dilated cardiomyopathy and a scapulohumeral-peroneal distribution of skeletal muscle involvement with concurrent tendon contractures. However, the same mutations can result in dilated cardiomyopathy with variable skeletal muscle involvement. Other dominant mutations most often leading to a change in the surface charge of the immunoglobulin fold in lamins A and C cause Dunnigan-type partial lipodystrophy, which has selective loss of subcutaneous fat from the extremities, excessive fat accumulation in the neck and face with the subsequent development of insulin resistance, diabetes mellitus, and liver steatosis. The autosomal recessive R298C LMNA mutation causes a Charcot–Marie–Tooth type 2 peripheral neuropathy characterized by a stocking-glove sensory neuropathy, an associated pes cavus foot deformity and additional variable features such as scoliosis. The de novo dominant G608G LMNA mutation causes the multisystem disease HGPS, with progeroid features including growth retardation, micrognathia, reduced subcutaneous fat, alopecia, osteoporosis, skin mottling, and premature vascular occlusive disease. Other rare dominant LMNA mutations also cause progeriod syndromes with similar features; the recessive R527H mutation causes mandibuloacral dysplasia, a disorder with a combination of progeroid features and partial lipodystrophy. Reprinted from Dauer and Worman66 with permission from Elsevier. (A color version of this figure is available in the online journal.)