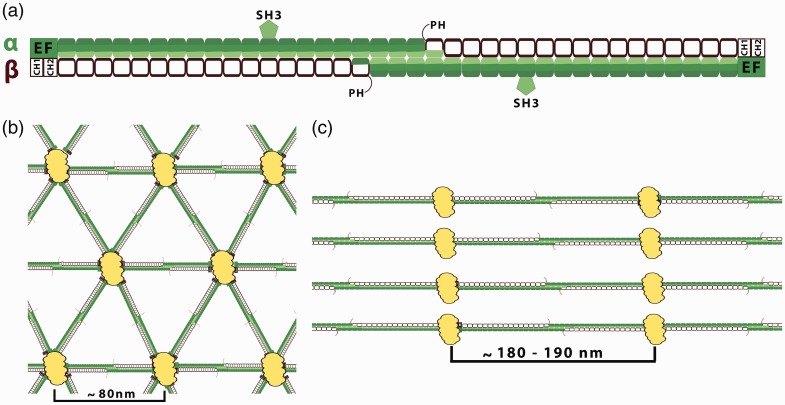
Figure 1.
Spectrin-based membrane skeleton. (a) Organization of the spectrin tetramers. Spectrins, as flexible long tetramers (∼200 nm length when fully extended) composed of α (filled segments) and β (empty segments) subunits associate side by side then form head-to-head dimer interactions. Each segment represents a 106-amino acid residue repeat unit (folded in a triple α-helical coiled-coil structure). The interconnections of spectrin repeats are thought to be closely associated with spectrin flexibility. α-Spectrin contains 21 repeats plus a single C-helix at the N terminus. A 60 amino acid residues fragment of the ninth repeat of the α-subunit represents an SH3 domain. β-Spectrin consists of 16 repeats plus a partial repeat at the C-terminus which contains just two A and B helices. Marked spectrin domains: PH: pleckstrin homology domain which is present only in “long” carboxyl end isoforms of the β-spectrin domain—except βI isoform; EF: EF-hand domain (calcium binding). Actin and protein 4.1R binding domain (2 CH domain) is located at the N-terminal end of the β-spectrin. (b) The quasi-triangle network of the erythrocytes spectrin-based skeleton. Spectrin tetramers are connected by junctional complexes (containing actin filaments, adducing, tropomodulin, and protein 4.1). The edge length of this network is ∼80 nm.10 The spectrin-based skeleton of resting erythrocytes is in a relaxed state what may be functionally helpful for the dynamics fully reversible deformations of the spectrin skeleton during circulation. (c) Periodicity of membrane skeleton in neuronal axons, where spectrin heterotetramers are connected to actin-based junctional complexes. The spectrin tetramers are spaced along the axon with periodicity of approximately 180–190 nm. This value agrees with the extended length of spectrin tetramers. The synergistic arrangement of bundling spectrin tetramers by actin rings in the same direction may increase the rigidity of spectrin tetramers. The lengths of spectrin tetramers in neuronal cells suggest that spectrin is under constant tensile stress. This force may be provided by the microtubule and neurofilament cytoskeletal systems that jam-pack inside neuronal processes, which are absent in erythrocytes.10(A color version of this figure is available in the online journal.)