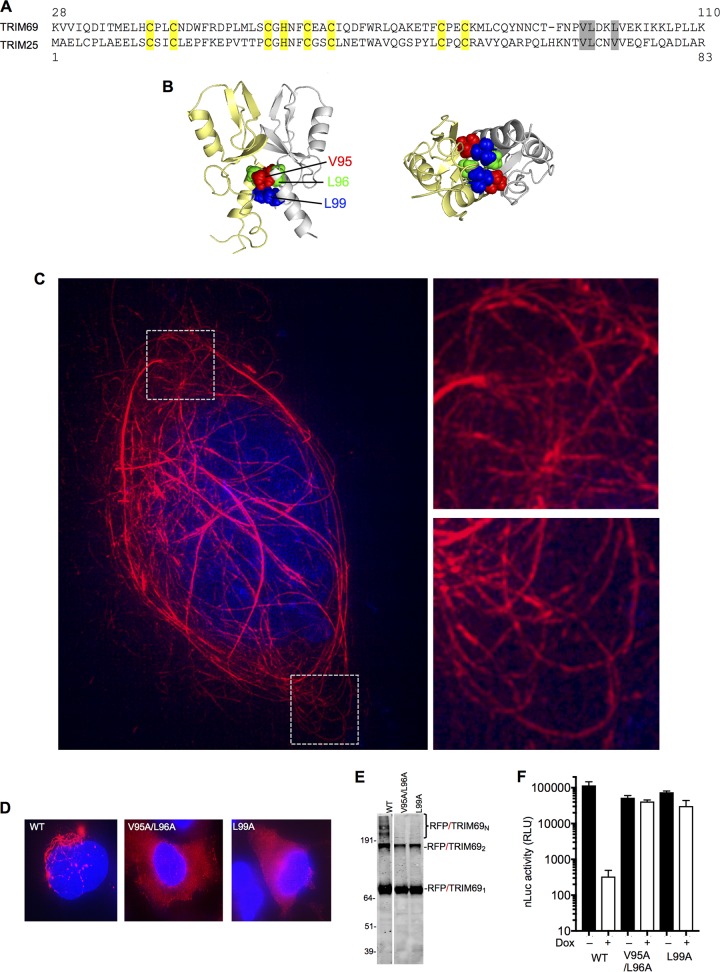
FIG 4.
Requirement for TRIM69 multimerization for antiviral activity. (A) Alignment of TRIM69 and TRIM25 RING domains. Yellow, zinc-coordinating residues; gray, mutated residues at the dimer interface. (B) Crystal structure of dimeric TRIM25 RING domain indicating amino acids at the dimer interface: V68, L69, and V72 are analogous to V95, L96, and L99 in TRIM69. (C) 3-D–SIM image of an mScarlet-TRIM69 expressing cell, with expanded views of the boxed areas. (D) Deconvolution microscopic images of WT and mutant TagRFP/TRIM69 fusion proteins expressed in doxycycline-inducible HT1080 cells. (E) Western blot analysis of WT and mutant TagRFP/TRIM69 fusion proteins following treatment of cells with EGS cross-linker prior to cell lysis. (F) VSVIND(nLuc) replication (luciferase activity in RLU) in HT1080 cells stably transduced with lentiviral vectors containing doxycycline-inducible expression cassettes for WT and mutant TagRFP/TRIM69 proteins. Mean values ± SD are shown.