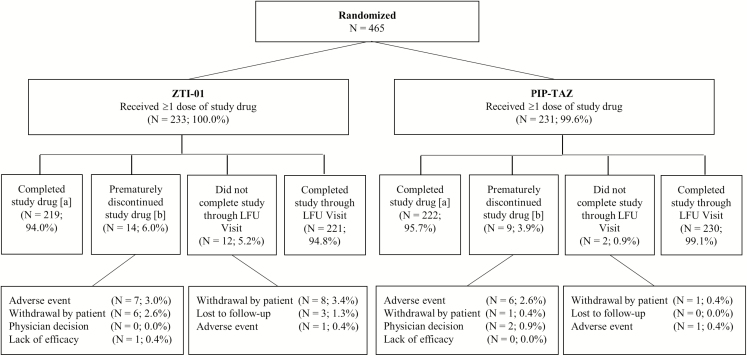
Figure 1.
Study design. N: Number of patients in the ITT population. Percentages were calculated using the number of patients in the ITT population as the denominator. A, Completed study drug was defined as completing 7 to 14 days of treatment. B, Only included patients who received study drug. Patient 1401–16 had an adverse event and did not receive study drug. Abbreviations: ITT, intent-to-treat; LFU, late follow-up; PIP-TAZ, piperacillin-tazobactam; TOC, test of cure; ZTI-01, fosfomycin for injection.