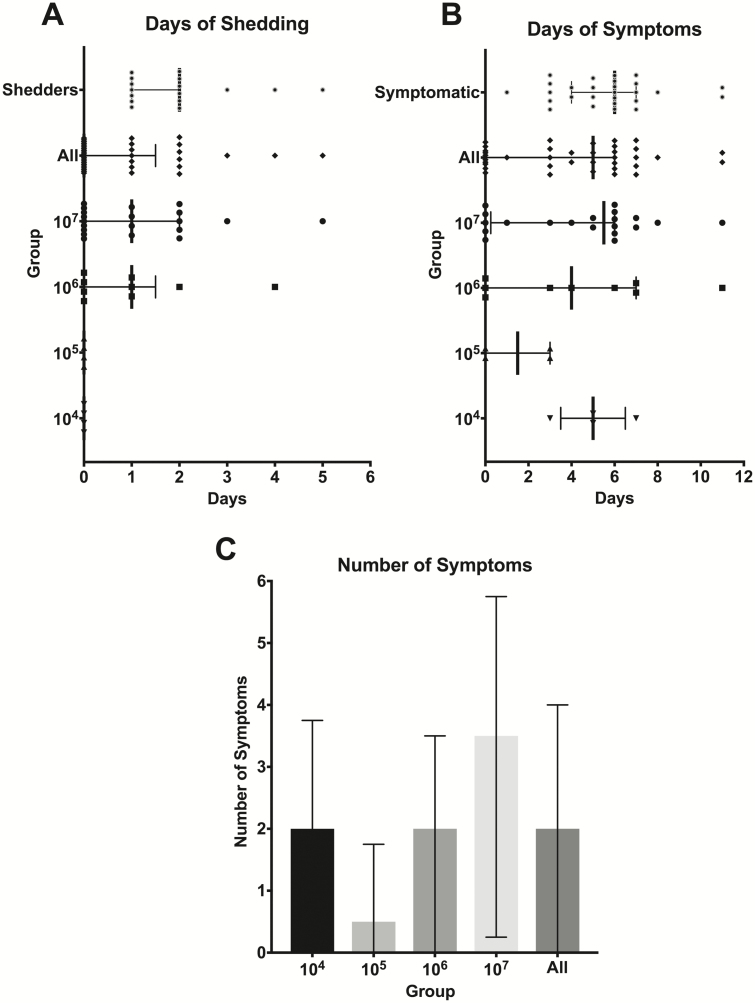
Figure 3.
Disease severity measures, by days of shedding and symptoms and by number of symptoms. A, Participants underwent daily nasal washes after challenge to identify the presence of influenza A(H3N2) after challenge. There was no viral shedding in the lowest 50% tissue culture infectious dose groups. The median duration overall for all shedders was 2 days and the median duration of viral shedding in the 2 highest TCID50 dose groups was 1 day. B, Study clinicians assessed clinical symptoms daily. Participants in all TCID50 dose groups experienced influenza symptoms with a median duration of 5 days for all participants. C, The highest number of symptoms occurred among the highest TCID50 dose group with a median of 3.5 symptoms over the study period. Lines represent medians with error bars representing the interquartile range. Abbreviations: TCID50, 50% tissue culture infectious dose.