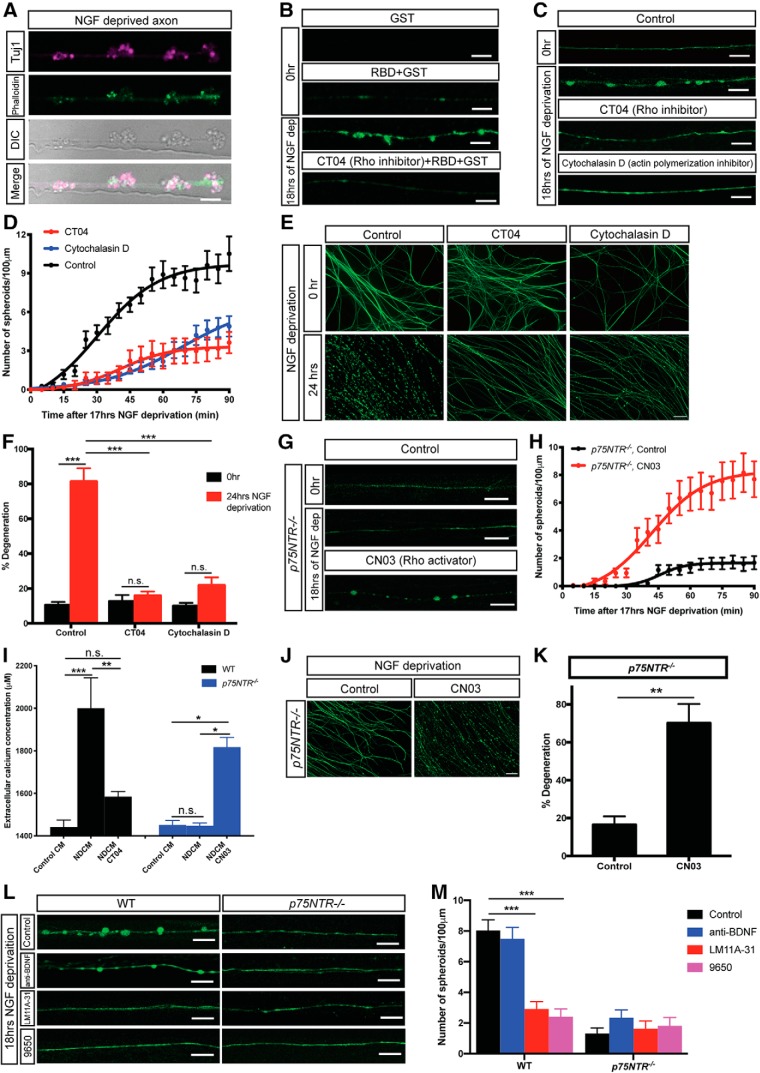
Figure 6.
p75NTR-Rho signaling is required for axonal spheroid formation. A, Representative axons/spheroids visualized for β3-tubulin (Tuj1), Phalloidin, and DIC after 18 h of NGF deprivation. Scale bar, 5 μm. B, Representative images of WT sympathetic axons immunostained for GST tag with or without NGF deprivation. All images, except the first one, show axons incubated with Rhotekin-RBD GST-fusion protein that binds active Rho proteins after fixation. Bottom, Axon was NGF-deprived and treated with 1 μg/ml Rho inhibitor CT04 for 3 h. Scale bar, 5 μm. C, Fluo4-AM calcium imaging of WT sympathetic axons with or without drug treatment. For the CT04 group, WT axons were incubated in SCG media containing 1 μg/ml Rho inhibitor CT04, for 2 h before 17 h of NGF deprivation. For the cytochalasin D group, WT axons were incubated in SCG media containing 10 μg/ml actin polymerization inhibitor for 2 h before 17 h of NGF deprivation. Scale bar, 10 μm. D, Quantification of axonal spheroid number per 100 μm of WT sympathetic axons at indicated time points after 17 h of NGF deprivation in the absence and presence of CT04 or cytochalasin D. Total number of n = 9 (Control), n = 8 (CT04), and n = 22 (cytochalasin D) axons from cultured neurons harvested from three independent litters were quantified. Nonlinear regression curves were drawn according to the Hill equation. E, F, Representative images (E) and quantification (F) of WT distal sympathetic axons immunostained for β3-tubulin in the absence and presence of CT04 or cytochalasin D. Scale bar, 50 μm. Compared with 0 h, p < 0.0001, n = 3 for Control, 24 h; p = 0.9256, n = 7 for CT04, 24 h; p = 0.1058, n = 6 for cytochalasin D, 24 h (two-way ANOVA with Sidak's multiple-comparisons test). Compared with Control, 24 h, p < 0.0001 for CT04, 24 h; p < 0.0001 for cytochalasin D, 24 h (two-way ANOVA with Dunnett's multiple-comparisons test). G, Fluo4-AM calcium imaging of p75NTR−/− sympathetic axons grown in the presence or absence of NGF with or without CN03 treatment. For the CN03 group, p75NTR−/− axons were incubated in SCG media containing 1 μg/ml Rho activator CN03 for 2 h before 17 h of NGF deprivation. Scale bar, 10 μm. H, Quantification of number of spheroids per 100 μm of p75NTR−/− sympathetic axons at indicated time points after 17 h of NGF deprivation in the absence and presence of CN03. Individual axons were counted: n = 16 (p75NTR−/−, Control) and n = 12 (p75NTR−/−, CN03) axons from three independent replicates. Nonlinear regression curves were drawn according to the Hill equation. I, Measurement of extracellular calcium concentration of untreated and NGF-deprived conditioned media in the presence and absence of CT04 or CN03 collected from WT (black) and p75NTR−/− (blue) sympathetic axons. Compared with WT, Control CM (n = 4), p < 0.0001, n = 4 for WT, NDCM; p = 0.4701, n = 6 for WT, NDCM CT04. Compared with WT NDCM, p = 0.0004 for WT, NDCM CT04. Compared with p75NTR−/−, Control CM (n = 4), p > 0.9999, n = 5 for p75NTR−/−, NDCM; p = 0.0088, n = 3 for p75NTR−/−, NDCM CN03. Compared with p75NTR−/−, NDCM, p = 0.0055 for p75NTR−/−, NDCM CN03 (one-way ANOVA with Tukey's multiple-comparisons test). J, K, Representative images (J) and quantification (K) of p75NTR−/− distal sympathetic axons immunostained for β3-tubulin after treatment with or without CN03 for 5 h. All cell cultures were pretreated with 12 h of NGF deprivation. Scale bar, 50 μm. p = 0.0006, n = 3 for CN03 and n = 7 for control (unpaired t test). L, M, Fluo4-AM calcium imaging (L) and spheroid number per 100 μm of WT and p75NTR−/− sympathetic axons (M) after 18 h of NGF deprivation in the absence and presence of 20 μg/ml anti-BDNF, 2 ng/ml p75NTR ligand/functional blocker LM11A-31, or 9650 immune serum (1:100). Scale bar, 10 μm. Individual axons were counted: n = 15 (WT, Control), n = 16 (WT, anti-BDNF), n = 37 (WT, LM11A-31), n = 22 (WT, 9650), n = 16 (p75NTR−/−, Control), n = 19 (p75NTR−/−, anti-BDNF), n = 18 (p75NTR−/−, LM11A-31), and n = 16 (p75NTR−/−, 9650) axons from three independent replicates. Significance is determined by two-way ANOVA with Dunnett's multiple-comparisons test. Data are mean ± SEM. *p < 0.05, **p < 0.001, ***p < 0.0001.