Abstract
Collateralization is an important way for patients with coronary heart disease to supply blood flow to the ischemic area. At present, research on the mechanism of collateral circulation mainly focuses on the inflammatory response. Monocytes are the kernel of inflammatory response during arteriogenesis. Therefore, we reviewed the recent developments in this field in terms of the dynamic changes of monocytes during collateralization. We searched and scanned PubMed for the following terms until November 2018: collateral, collateralization, monocyte, macrophage, and arteriogenesis. Articles were obtained and examined to figure out the dynamics of monocytes in the progress of collateralization. Substantial research shows that recruitment, infiltration, and phenotypic transformation of monocytes can affect function in various ways, respectively. Mechanical or chemical factors that can produce effects on collateral development may be due partly to impact on dynamics of monocytes. Although mechanisms of dynamics of monocytes during arteriogenesis are not elucidated clearly, there is no doubt that deeper exploration of the underlying mechanisms will contribute to pharmaceutical development aiming for promoting collateral development.
Keywords: arteriogenesis, collateral, collateralization, macrophage, monocyte
1. INTRODUCTION
The present treatment for coronary artery disease mainly includes medication and drug therapy combined with coronary revascularization. However, the rate of restenosis after stent implantation is still as high as 10% to 20%,1 and approximately 20% of patients with coronary artery diffuse multivessel disease are unable to tolerate percutaneous coronary intervention or coronary artery bypass grafting.2 Then, does any strategy effectively alleviate myocardial ischemic symptoms for those patients? Collateral circulation might be the answer.
Many clinical studies show that well‐established collateral circulation can effectively protect cardiac muscle from ischemia and improve patients’ prognoses.3 Collateral vessel refers to the tiny anatomical microvessels that bridge the severe stenosis or occlusion between the proximal and distant parts of the same blood vessel or between different blood vessels, with a diameter of 20‐350 μm, which can provide blood supply to the ischemic regions and confine myocardial infarct size.4 Related studies have shown that good collateral circulation plays a role in protecting myocardium from acute myocardial infarction and chronic myocardial ischemia.5, 6, 7, 8 A meta‐analysis enrolling 6529 participants from several studies showed that patients with high collateralization had a 36% reduction in death risk compared with patients with low collateralization.9 Therefore, the establishment of coronary collateral circulation is of great significance for the prognosis of coronary artery disease.
The development of collateral circulation refers to the remodeling and enlargement of pre‐existing collateral vessels.10 It is currently believed that when the coronary artery is stenotic or occluded, a pressure gradient between both ends of a pre‐existing collateral vessel occurs, which results in an increase in collateral blood flow, thereby increasing the shear stress, which activates endothelial cells.11, 12, 13 The expression of adhesion molecules and chemokines in activated endothelial cells is subsequently upregulated, which promotes aggregation, adhesion, and migration of monocytes into the blood vessel wall to induce a local inflammatory response.14, 15 After migrating into the blood vessel wall, monocytes differentiate into macrophages and secrete matrix metalloproteinases to dissolve extracellular matrix and basement membrane.16, 17, 18 Then, various cytokines and growth factors promote phenotype transformation and proliferation of endothelial cells and smooth muscle cells, thus resulting in arterial outward remodeling into mature collateral vessels.4, 19, 20
Current theory suggests that the involvement of inflammation during the establishment of the collateral circulation is essential, while monocytes are at the core of the inflammatory responses during arteriogenesis (Figure 1). Therefore, we reviewed the recent studies of monocytes participating in the development of collateral circulation.
Figure 1.
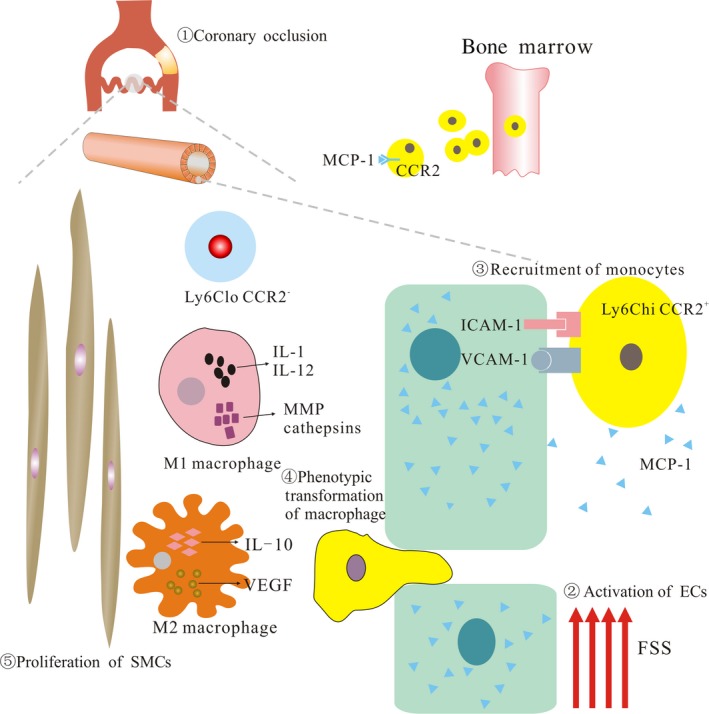
Collateral circulation. During the process of (1) chronic coronary arterial occlusion, the increase of fluid shear stress (FSS) owing to augmentation of collateral flow due to the precipitous pressure gradient between a stenosis or occlusion can (2) activate endothelial cells (ECs). (3) Then, activated ECs secrete monocyte chemoattractant protein (MCP‐1) to recruit monocytes from bone marrow. (4) After recruitment, infiltration, and invasion of monocytes, relating stimulative factors facilitate phenotypic transformation of macrophages. (5) Simultaneously, various growth factors or cytokines promote proliferation and migration of ECs and smooth muscle cells (SMCs), resulting in arterial outward remodeling into mature collateral vessels. IL, interleukin; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor
2. INVOLVEMENT OF MONOCYTES IN ARTERIOGENESIS
Studies on the involvement of monocytes in arteriogenesis can be traced back to the 1970s. In 1976, Schaper et al. used scanning and transmission electron microscopy to find that the early endothelial cells during arteriogenesis have significant longitudinal protrusions in the canine model of chronic coronary artery occlusion, and there were a large number of monocytes adhered to the surface of endothelial cells.21 Subsequently, they demonstrated the recruitment of monocytes in the collateral artery during arteriogenesis in rabbit and murine hindlimb ischemia models.22, 23, 24, 25 It has also been found that mice with monocytes deficiency have poorer blood flow recovery and arterial formation after hindlimb femoral artery ligation than those in a control group.26 This series of studies focusing on monocytes has expanded horizons of the mechanism of arteriogenesis, confirming that inflammation participates in arteriogenesis and plays an important role.
3. ORIGIN OF MONOCYTES
Controversies about the origins of monocytes have existed for decades. In the myocardial infarction model, substantial studies have confirmed that recruited monocytes are mainly derived from bone marrow.27 In recent years, studies have also found that, as a monocytes reservoir, the spleen also provides a large number of monocytes during myocardial remodeling after infarction.28 However, some researchers found that in a hindlimb ischemia model, monocytes recruited to collateral vessels were not from the spleen.29 Related studies have found that the formation of collateral circulation in the model of hindlimb ischemia is significantly undermined after deletion of monocytes by liposome‐containing bisphosphonate and 5‐fluorouracil, which can suppress bone marrow.30 Interestingly, in the rat hindlimb ischemia model, the authors used cyclophosphamide to remove circulating monocytes but found no effect on the number of monocytes recruited to the collateral arteries. Therefore, the authors believed that circulating monocytes are not involved in arteriogenesis, but that local proliferation of tissue‐derived macrophages plays an important role in the establishment of collateral arteries.31 Due to the technical limits in achieving space and time‐specific knockout of monocytes, controversies over the origin of monocytes will remain. However, it still can be speculated that both bone‐marrow‐derived and local proliferating monocytes may exert great effects on the arteriogenic inflammatory response.32
4. RECRUITMENT OF MONOCYTES
As is known to all, monocytes recruitment requires the existence of chemokines. In 1997, Schaper et al. reported that monocyte chemoattractant (MCP‐1) injected into the ischemic rabbit and porcine hindlimb can promote collateral artery development and monocytes accumulation around the arterial walls.33, 34 Therefore, they believed that enhanced monocyte recruitment and activation is of great significance in the process of collateral artery development. In 2003, van Royen et al. also found that MCP‐1 exerts a significant promoting effect on the collateral growth in ApoE (−/−) mice.35 Simultaneously they found that blood flow recovery and monocytes recruitment are worse in MCP‐1 knockout mice compared with wild‐type mice. Therefore, recruitment of monocytes is the prerequisite of high collateralization, which elucidates the reason why monocytes accumulate on targeted vessels.
In recent years, more and more studies have found that the reason why many physical and chemical factors can affect arteriogenesis is their important roles in the recruitment and migration of monocytes.32 Studies have shown that stretch generated by blood flow acting on vessels can promote the expression of ephrinB2 in endothelial cells, which may play a key role in arteriogenesis, while the truncated variant of ephrinB2 can significantly inhibit mononuclear cells from migrating into the blood vessel wall.36 Recently, Buschmann et al. reported that high levels of fluid shear stress (FSS) in a porcine arteriovenous fistula model promoted the adhesion and migration of monocytes by inducing the expression of MCP‐1 and tissue inhibitors of metalloproteinases, thereby promoting pre‐existing collateral remodeling.37 Recently, Laban et al. showed that VASP is an important regulator of monocytes recruitment migration and polarization during ischemia and revascularization and confirmed that VASP plays an important role in the transport of chemokine receptors.38 The recruitment and migration of monocytes is a carefully orchestrated process that relies on the coordination of cytokine concentrations, adhesion molecule expression, and monocyte actin dynamics, which directly affect the role of monocytes in arteriogenesis. Therefore, it is especially necessary to explore the relevant mechanisms of monocytes recruitment and migration.
5. MONOCYTE PHENOTYPE
Monocytes are mainly divided into two subsets according to the differences in expression of their cell surface markers: inflammatory (Ly6ChiCCR2+CX3CR1low) and tissue‐resident (Ly6ClowCCR2–CX3CR1high), which correspond to CD14+CD16+ and CD14lowCD16– subsets in humans, respectively.39, 40 In the early days after the start of arterial occlusion and tissue ischemia, the collateral vessel wall is predominantly infiltrated by Ly6Chi monocytes, whereas subsequently (about 5‐7 days after the onset of ischemia) Ly6Clo monocytes predominate the vessel wall.29, 41 Ly6Chi monocytes secrete various proteases, such as matrix metalloproteinases, cathepsins, and elastase, while Ly6Clo monocytes mainly secrete growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor.42, 43 The subpopulation of monocytes infiltrating into vascular wall changes over time, and its corresponding function during arteriogenesis also varies.
Studies have already suggested that although monocytes can phagocytose apoptotic cell debris, the role of different subsets of monocytes in ischemic tissue and collateral growth has not been elucidated. Nowadays, most scholars believe that Ly6Chi monocytes are the main participants in proinflammatory response, such as matrix degradation, while Ly6Clo monocytes promote collateral growth by secreting growth factors.44, 45 Numerous studies have shown that increased recruitment of Ly6Clo monocytes can significantly boost arteriogenesis and remodeling of microvascular networks.41 However, the study of a hindlimb ischemia model showed that transplantation of Ly6Chi monocytes can significantly promote the revascularization process, while Ly6Clo monocytes have no such effect.29 On account of the specific role of Ly6Chi and Ly6Clo monocytes, in‐depth explorations are still expected.
6. MACROPHAGE PHENOTYPIC TRANSFORMATION
The research found that M1/M2 macrophages have a significant impact on collateral development.46, 47, 48 It is currently believed that M1 macrophages are mainly derived from Ly6Chi monocytes, which exert an inflammatory effect and express iNOS and a large variety of pro‐inflammatory cytokines, such as interleukin (IL)‐1 and IL‐12.48 M2 macrophages are mainly derived from the differentiation of Ly6Clo monocytes, which primarily produce anti‐inflammatory effects, and express a large number of cytokines, such as IL‐10, arginase‐1, VEGF, and growth factors, to promote arteriogenesis.48 However, studies have also found that Ly6Chi monocytes can also differentiate into M2 macrophages.49, 50 This research suggests that the phenotype of M1/M2‐type macrophages has strong plasticity, which makes it possible for medication to promote the establishment of collateral via inducing macrophage phenotypic transformation.
Most studies have shown that increasing the polarization of M2 macrophages can significantly enhance the blood flow recovery of hindlimb ischemia model and promote collateral growth.51, 52 Therefore, many scholars have carried out research on how to promote the polarization of M2 macrophages from different perspectives. In 2011, Takeda et al. found that Phd2 (also known as Egln1) haplodeficient (Phd2(+/−)) mice had improved collateral artery and arteriogenesis by increasing the number of settled M2 macrophages and the release of arteriogenic factors.53 Ganta et al. showed that relative to wild‐type mice, miR106b‐93‐25 cluster‐deficient mice had significantly lower angiogenesis and arteriogenesis after femoral artery ligation, and their M1 macrophages were significantly increased. However, they also found that miR93 can directly act on interferon regulator‐9, and regulate the expression of immunoresponsive gene‐1 (IRG‐1)‐itaconic acid to facilitate the polarization of M2 macrophages in ischemic tissues, thereby promoting angiogenesis, arterial growth, and blood flow recovery in ischemic hindlimb.54 Babu et al. showed that hypomethylation of M1‐type inflammatory genes and hypermethylation of M2‐type anti‐inflammatory pro‐angiogenic genes in hyperlipidemia or type 2 diabetic mice suggested that hyperlipidemia or hyperglycemia is one of the causes leading to impaired arterial growth and angiogenesis in ischemic hindlimb.55 At present, researchers are trying to explain the polarization of M1/M2 macrophages from the perspective of epigenetics and proteomics, but the relevant mechanisms have not yet been clearly stated and we still lack effective means to regulate the polarization of M1/M2 macrophages.
7. CONCLUDING REMARKS
As we know, the establishment of collateral circulation is an important means of compensating blood flow supply for the ischemic tissue, which can effectively alleviate ischemic damage and improve prognosis of patients with coronary artery disease.56, 57 However, in current clinical medicine, there are still no efficacious drugs to promote development of collateral circulation. Therefore, deeper exploration of the mechanism of collateral circulation is of great significance for boosting the progress of treatment of ischemic and hypoxic diseases.58
Monocytes recruitment to the collateral artery was discovered decades ago in animal experiments. At the same time, relevant clinical studies have proved that serum MCP‐1 and Omentin‐1 level are significantly higher in patients with better collateralization, which may be predictive markers of good collateral circulation.59, 60 Nevertheless, a recent study has found that blood monocytes in patients with developed collateral are remarkably lower than those in patients with poor collateral and an increased lymphocyte/monocyte ratio predicts well‐developed coronary collateral.61 In chronic occlusion patients, the expression of miRNA339‐5p in monocytes of low collateral capacity patients is significantly decreased.62 Hence, it may be inferred that monocyte or its relevant factors are the potential markers of good/poor collateral.
In recent years, new discoveries about collateral circulation have been found in many fields. From the perspective of epigenetics, scholars have found that miRNA‐15b, miRNA‐93, and miRNA‐352 can be mediators to regulate collateral formation.63, 64 In 2011, W. Schaper's group firstly revealed that the formation of collateral vessels was simultaneously innervated.65 Meanwhile, they proved the elevated expression of various cell growth factors, such as acidic fibroblast growth factor, basic fibroblast growth factor, and VEGFA, during collateral vessel formation.66 Recently, Schirmer et al. revealed that monocytic nitric oxide plays a crucial role in the process of collateral artery growth.67 Besides, Yıldırım et al. show that galectin‐2 can suppress arteriogenesis through inducing proinflammatory phenotype of monocytes.68, 69
Local inflammation of the collateral arteries is critical for arteriogenesis. Monocytes take a dynamic part in affecting inflammation and anti‐inflammation as a kernel during local inflammation. At present, the application of VEGF, granulocyte‐macrophage colony‐stimulating factor, granulocyte colony‐stimulating factor, and so forth in clinical trials cannot effectively promote the establishment of collateral circulation in patients with coronary heart disease.70, 71, 72 Therefore, more profound understanding of monocytes/macrophages subset transformation, chemotactic adhesion factors, and related mechanisms during the growth of collateral artery is of great significance for the study of pharmacotherapy, which aims to effectively promote collateral development.
CONFLICT OF INTEREST
There are no conflicts of interest.
ACKNOWLEDGMENTS
We acknowledge grants from the National Natural Science Foundation of China (81570239, 81770301, and 81822004).
Liao L‐S, Bai Y‐P. The dynamics of monocytes in the process of collateralization. Aging Med. 2019;2:50‐55. 10.1002/agm2.12054
REFERENCES
- 1. Alfonso F, Pérez‐Vizcayno MJ, Cárdenas A, et al. A prospective randomized trial of drug‐eluting balloons versus everolimus‐eluting stents in patients with in‐stent restenosis of drug‐eluting stents: the RIBS IV Randomized Clinical Trial. J Am Coll Cardiol. 2015;66(1):23‐33. [DOI] [PubMed] [Google Scholar]
- 2. Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J. 2013;34(34):2674‐2682. [DOI] [PubMed] [Google Scholar]
- 3. Helfant RH, Vokonas PS, Gorlin R. Functional importance of the human coronary collateral circulation. New Engl J Med. 1971;284(23):1277‐1281. [DOI] [PubMed] [Google Scholar]
- 4. Pries AR, Badimon L, Bugiardini R, et al. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the Working Group on Coronary Pathophysiology and Microcirculation. Eur Heart J. 2015;36(45):3134‐3146. [DOI] [PubMed] [Google Scholar]
- 5. Meier P, Schirmer SH, Lansky AJ, Timmis A. The collateral circulation of the heart. BMC Med. 2013;11(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Regieli JJ, Jukema JW, Nathoe HM, et al. Coronary collaterals improve prognosis in patients with ischemic heart disease. Int J Cardiol. 2009;132(2):257‐262. [DOI] [PubMed] [Google Scholar]
- 7. Perez‐Castellano N, Garcia EJ, Abeytua M, et al. Influence of collateral circulation on in‐hospital death from anterior acute myocardial infarction. J Am Coll Cardiol. 1998;31(3):512‐518. [DOI] [PubMed] [Google Scholar]
- 8. Meier P, Gloekler S, Zbinden R, et al. Beneficial effect of recruitable collaterals: a 10‐year follow‐up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116(9):975‐983. [DOI] [PubMed] [Google Scholar]
- 9. Meier P, Hemingway H, Lansky AJ, Knapp G. The impact of the coronary collateral circulation on mortality: a meta‐analysis. Eur Heart J. 2012;33(5):614‐621. [DOI] [PubMed] [Google Scholar]
- 10. Simons M, Eichmann A. Molecular controls of arterial morphogenesis. Circ Res. 2015;116(10):1712‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104(1):5‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoefer IE, den Adel B, Daemen MJAP. Biomechanical factors as triggers of vascular growth. Cardiovasc Res. 2013;99(2):276‐283. [DOI] [PubMed] [Google Scholar]
- 13. Zhou J, Li YS, Chien S. Shear stress‐initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34(10):2191‐2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoefer IE, van Royen N, Rectenwald JE, et al. Arteriogenesis proceeds via ICAM‐1/Mac‐1‐ mediated mechanisms. Circ Res. 2004;94(9):1179‐1185. [DOI] [PubMed] [Google Scholar]
- 15. Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10(1):83‐97. [DOI] [PubMed] [Google Scholar]
- 16. Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res. 2004;95(5):449‐458. [DOI] [PubMed] [Google Scholar]
- 17. Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Bioch Bioph Sin. 2008;40(8):681‐692. [PubMed] [Google Scholar]
- 18. Zimarino M, D'Andreamatteo M, Waksman R, Epstein SE. The dynamics of the coronary collateral circulation. Nat Rev Cardiol. 2014;11(4):191‐197. [DOI] [PubMed] [Google Scholar]
- 19. Buschmann IR, Busch HJ, Mies G, Hossmann KA. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte‐macrophage colony‐stimulating factor. Circulation. 2003;108(5):610‐615. [DOI] [PubMed] [Google Scholar]
- 20. Nagy JA, Dvorak AM, Dvorak HF. VEGF‐A 164/165 and PlGF: roles in angiogenesis and arteriogenesis. Trends Cardiovas Med. 2003;13(5):169‐175. [DOI] [PubMed] [Google Scholar]
- 21. Schaper J, König R, Franz D, Schaper W. The endothelial surface of growing coronary collateral arteries. Intimal margination and diapedesis of monocytes. Virchows Arch A Pathol Anat Histol. 1976;370(3):193‐205. [DOI] [PubMed] [Google Scholar]
- 22. Tibor Z, Borja F, Sawa K, et al. Bone marrow‐derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94(2):230‐238. [DOI] [PubMed] [Google Scholar]
- 23. Arras M, Ito WD, Scholz D, Winkler B. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101(1):40‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scholz D, Ito W, Fleming I, et al. Ultrastructure and molecular histology of rabbit hind‐limb collateral artery growth (arteriogenesis). Virchows Arch. 2000;436(3):257‐270. [DOI] [PubMed] [Google Scholar]
- 25. Scholz D, Ziegelhoeffer T, Helisch A, et al. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34(7):775‐787. [DOI] [PubMed] [Google Scholar]
- 26. Bergmann CE, Hoefer IE, Benjamin M, et al. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukocyte Biol. 2006;80(1):59‐65. [DOI] [PubMed] [Google Scholar]
- 27. Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35(5):1066‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laan AMVD, Horst ENT, Ronak D, et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2014;35(6):376‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cochain C, Rodero MJ, Recalde A, et al. Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post‐ischaemic neovascularization. Cardiovasc Res. 2010;88(1):186‐195. [DOI] [PubMed] [Google Scholar]
- 30. Pipp F, Heil M, Issbrücker K, et al. VEGFR‐1‐selective VEGF homologue PlGF is arteriogenic. Circ Res. 2003;92(4):378‐385. [DOI] [PubMed] [Google Scholar]
- 31. Khmelewski E, Becker A, Meinertz T, Ito WD. Tissue resident cells play a dominant role in arteriogenesis and concomitant macrophage accumulation. Circ Res. 2004;95(6):56‐64. [DOI] [PubMed] [Google Scholar]
- 32. Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol. 2012;3:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein‐1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res. 1997;80(6):829‐837. [DOI] [PubMed] [Google Scholar]
- 34. Hoefer IE, van Royen N, Buschmann IR, Piek JJ, Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49(3):609‐617. [DOI] [PubMed] [Google Scholar]
- 35. van Royen N, Hoefer I, Ttinger BM, et al. Local monocyte chemoattractant protein‐1 therapy increases collateral artery formation in apolipoprotein E‐deficient mice but induces systemic monocytic CD11b expression, neointimal formation, and plaque progression. Circ Res. 2003;92(2):218‐225. [DOI] [PubMed] [Google Scholar]
- 36. Korff T, Braun J, Pfaff D, Augustin HG. Role of ephrinB2 expression in endothelial cells during arteriogenesis: impact on smooth muscle cell migration and monocyte recruitment. Blood. 2008;112(1):73‐81. [DOI] [PubMed] [Google Scholar]
- 37. Buschmann EE, Lee EJ, Jacobi D, et al. Induction of extracranial arteriogenesis by an arteriovenous fistula in a pig model. Atherosclerosis. 2018;272:87‐93. [DOI] [PubMed] [Google Scholar]
- 38. Laban H, Weigert A, Zink J, et al. VASP regulates leukocyte infiltration, polarization, and vascular repair after ischemia. J Cell Biol. 2018;217(4):1503‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geissmann F, Jung S, Dan RL. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71‐82. [DOI] [PubMed] [Google Scholar]
- 40. Auffray C, Fogg DME, Senechal B, et al. CX3CR1 + CD115 + CD135 + common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206(3):595‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matthias N, Swirski FK, Elena A, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037‐3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jaipersad AS, Lip GYH, Stanley S, Eduard S. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 43. Capoccia BJ, Gregory AD. Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein‐1‐dependent fashion. J Leukocyte Biol. 2008;84(3):760‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ziegler‐Heitbrock HW, Fingerle G, Str Bel M, et al. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23(9):2053‐2058. [DOI] [PubMed] [Google Scholar]
- 45. Axel S, Heine GH, Stefan B, et al. CD14 + CD16 + monocytes in coronary artery disease and their relationship to serum TNF‐alpha levels. Thromb Haemost. 2004;91(02):419‐424. [DOI] [PubMed] [Google Scholar]
- 46. Seaman SA, Cao Y, Campbell CA, Peirce SM. Macrophage recruitment and polarization during collateral vessel remodeling in murine adipose tissue. Microcirculation. 2016;23(1):75‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu H, Zhang M, Liu Z, et al. AMP‐activated protein kinase α1 in macrophages promotes collateral remodeling and arteriogenesis in mice in vivo. Arterioscler Thromb Vasc Biol. 2016;36(9):1868‐1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sala AL, Pontecorvo L, Agresta A. Regulation of collateral blood vessel development by the innate and adaptive immune system. Trends Mol Med. 2012;18(8):494‐501. [DOI] [PubMed] [Google Scholar]
- 49. Kiavash M, Damya L, Conny G, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728‐5739. [DOI] [PubMed] [Google Scholar]
- 50. Shuei Liong L, Casta OAP, Nowlin BT, Lupher ML. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183(10):6733‐6743. [DOI] [PubMed] [Google Scholar]
- 51. Troidl C, Jung G, Troidl K, et al. The temporal and spatial distribution of macrophage subpopulations during arteriogenesis. Curr Vasc Pharmacol. 2013;11(1):5‐12. [PubMed] [Google Scholar]
- 52. Lasch M, Caballeromartinez A, Troidl K, Schloegl I. Arginase inhibition attenuates arteriogenesis and interferes with M2 macrophage accumulation. Lab Invest. 2016;96(8):830‐838. [DOI] [PubMed] [Google Scholar]
- 53. Takeda Y, Costa S, Delamarre E, et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479(7371):122‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ganta VC, Choi MH, Kutateladze A, Fox TE, Farber CR, Annex BH. A microRNA93‐interferon regulatory factor‐9‐immunoresponsive gene‐1‐itaconic acid pathway modulates M2‐like macrophage polarization to revascularize ischemic muscle. Circulation. 2017;135(24):2403‐2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Babu M, Durga Devi D, Mäkinen PI, et al. Differential promoter methylation of macrophage genes is associated with impaired vascular growth in ischemic muscles of hyperlipidemic and type 2 diabetic mice: a genome‐wide promoter methylation study. Circ Res. 2015;117(3):289‐299. [DOI] [PubMed] [Google Scholar]
- 56. Khand A, Fisher M, Jones J, Patel B. The collateral circulation of the heart in coronary total arterial occlusions in man: systematic review of assessment and pathophysiology. Am Heart J. 2013;166(6):941‐952. [DOI] [PubMed] [Google Scholar]
- 57. Christian S, Pascal M. Historical aspects and relevance of the human coronary collateral circulation. Curr Cardiol Rev. 2014;10(1):2‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Silvestre JS, Smadja DM, Levy BI. Postischemic revascularization: from cellular and molecular mechanisms to clinical applications. Physiol Rev. 2013;93(4):1743‐1802. [DOI] [PubMed] [Google Scholar]
- 59. Sahinarslan A, Kocaman SA, Topal S, Erçin U. The relation of serum monocyte chemoattractant protein‐1 level with coronary atherosclerotic burden and collateral degree in stable coronary artery disease. Turk Kardiyol Dern Ars. 2011;39(4):269‐275. [DOI] [PubMed] [Google Scholar]
- 60. Zhou JP, Tong XY, Zhu L, et al. Plasma omentin‐1 level as a predictor of good coronary collateral circulation. J Atheroscler Thromb. 2017;24(9):940‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kurtul A, Duran M. The correlation between lymphocyte/monocyte ratio and coronary collateral circulation in stable coronary artery disease patients. Biomark Med. 2017;11(1):43‐52. [DOI] [PubMed] [Google Scholar]
- 62. Hakimzadeh N, Elias J, Wijntjens GWM, et al. Monocytic microRNA profile associated with coronary collateral artery function in chronic total occlusion patients. Sci Rep. 2017;7(1):1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhu LP, Zhou JP, Zhang JX, et al. MiR‐15b‐5p regulates collateral artery formation by targeting AKT3 (protein kinase B‐3) highlights. Arterioscler Thromb Vasc Biol. 2017;37(5):957‐968. [DOI] [PubMed] [Google Scholar]
- 64. Guan Y, Cai B, Wu X, et al. microRNA‐352 regulates collateral vessel growth induced by elevated fluid shear stress in the rat hind limb. Sci Rep. 2017;7(1):6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Luo MY, Yang BL, Ye F, et al. Collateral vessel growth induced by femoral artery ligature is impaired by denervation. Mol Cell Biochem. 2011;354(1–2):219‐229. [DOI] [PubMed] [Google Scholar]
- 66. Wu S, Wu X, Zhu W, et al. Immunohistochemical study of the growth factors, aFGF, bFGF, PDGF‐AB, VEGF‐A and its receptor (Flk‐1) during arteriogenesis. Mol Cell Biochem. 2010;343(1–2):223‐229. [DOI] [PubMed] [Google Scholar]
- 67. Schirmer SH, Millenaar DN, Werner C, et al. Exercise promotes collateral artery growth mediated by monocytic nitric oxide. Arterioscler Thromb Vasc Biol. 2015;35(8):1862‐1871. [DOI] [PubMed] [Google Scholar]
- 68. Yıldırım C, Vogel DY, Hollander MR, et al. Galectin‐2 induces a proinflammatory, anti‐arteriogenic phenotype in monocytes and macrophages. PLoS ONE. 2015;10(4):e0124347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. van der Laan AM, Schirmer SH, de Vries MR, et al. Galectin‐2 expression is dependent on the rs7291467 polymorphism and acts as an inhibitor of arteriogenesis. Eur Heart J. 2012;33(9):1076‐1084. [DOI] [PubMed] [Google Scholar]
- 70. Seiler C, Pohl T, Wustmann K, et al. Promotion of collateral growth by granulocyte‐macrophage colony‐stimulating factor in patients with coronary artery disease: a randomized, double‐blind, placebo‐controlled study. Circulation. 2001;104:2012‐2017. [DOI] [PubMed] [Google Scholar]
- 71. Zbinden S, Zbinden R, Meier P, Windecker S. Safety and efficacy of subcutaneous‐only granulocyte‐macrophage colony‐stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1636‐1642. [DOI] [PubMed] [Google Scholar]
- 72. Meier P, Gloekler S, de Marchi S, et al. Myocardial salvage through coronary collateral growth by granulocyte colony‐stimulating factor in chronic coronary artery disease: a controlled randomized trial. Circulation. 2009;120:1355‐1363. [DOI] [PubMed] [Google Scholar]


