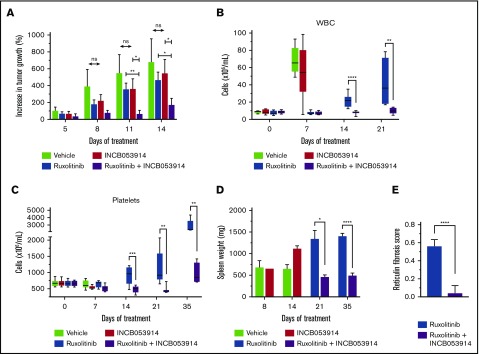
Figure 6.
INCB053914 synergizes with ruxolitinib to inhibit MPN model cell tumor formation and antagonizes the development of MPN that persists during ruxolitinib treatment of a murine MPN model. (A) CB17 SCID mice were subcutaneously injected with SET2 cells and tumors were allowed to form for 13 days. At that point, mice were randomly assigned to generate 4 cohorts with equal average tumor sizes. Mice were treated with vehicle (n = 10, starting tumor size mean ± standard error of the mean [SEM] = 129 ± 25 mm3), ruxolitinib (30 mg/kg) (n = 7; mean, 140 ± 25 mm3), INCB053914 (100 mg/kg) (n = 9; mean, 130 ± 28 mm3 mg/kg), or a combination of the 2 drugs (n = 10; mean, 128 ± 18 mm3) twice per day by oral gavage. The average percent increase in tumor size for each treatment is shown over time (mean ± SEM). A statistically significant difference between each monotherapy and the combination was observed. (B) The MPL-W515L bone marrow transplant mouse model of MPN was used to assess the effects of INCB053914 alone and in combination with ruxolitinib on the development of MPN in vivo. Mouse bone marrow cells were retrovirally infected with virus containing the MPL-W515L MPN-driving oncogene and injected into mice whose bone marrow was ablated with 5-fluorouracil. Blood cell counts were performed 7 days after transplantation, and on that day, 4 cohorts (n = 9 each) with equal mean platelet numbers were generated for treatment with vehicle (mean platelet count ± SEM = 673 ± 34 × 109/L), INCB053914 (100 mg/kg; mean platelet count, 673 ± 32 × 109/L), ruxolitinib (60 mg/kg; mean platelet count, 667 ± 29 × 109/L), and a combination of those doses of INCB053914 and ruxolitinib (mean platelet count, 665 ± 26 × 109/L) twice per day orally. Blood counts were then performed after 7, 14, 21, and 35 days of treatment. White blood cell (WBC) counts (B) and platelet counts (C) are shown over days of treatment (boxes indicate 25th to 75th percentile, whiskers indicate the range, and the horizontal line indicates the median). WBC counts at day 35 of treatment were not obtained because of machine error. (D) Spleen weights of vehicle- (n = 4) and INCB053914 monotherapy-treated (n = 1) animals were obtained when they became moribund on day 8 of treatment; the remaining vehicle- and INCB053914 monotherapy–treated animals were moribund on day 14 of treatment. Three mice each for the ruxolitinib monotherapy and the combination therapy cohorts were euthanized to assess disease progression (spleen weight) on day 21 of treatment. Treatment continued for 35 days total (day 42 following transplantation) at which point the experiment was stopped, all animals were euthanized, and spleens were weighed. Mean spleen weights are shown (± SEM) over time. (E) Scoring of reticulin bone marrow in ruxolitinib-treated mice and mice treated with the combination therapy (data points represent the average of the average of 3 fields of n = 4 mice ± SD). *P < .05; **P < .01; ***P < .001; ****P < .0001 by unpaired Student t test.