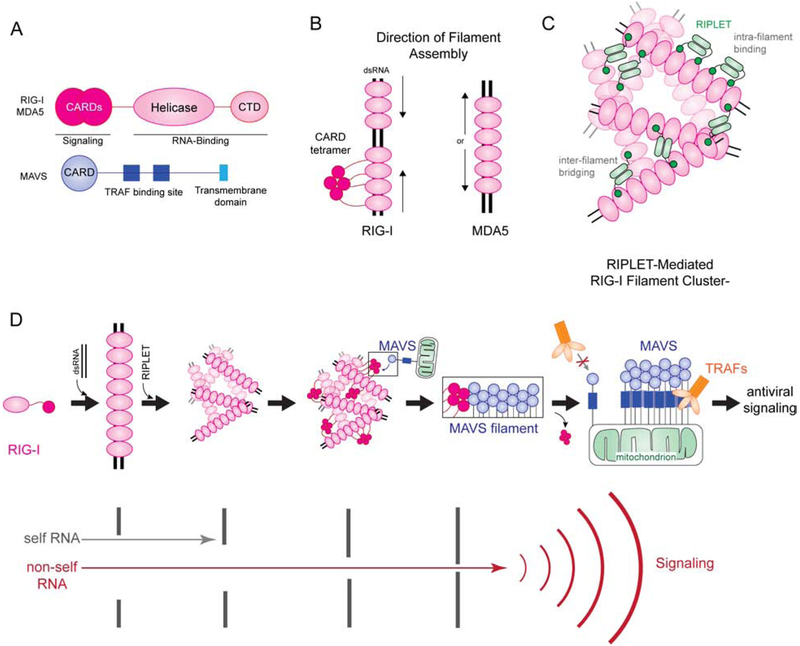
Figure 2.
Filament formation and higher order oligomerization of RIG-I-like receptors. A) Both RIG-I and MDA5 share the same domain architecture, consisting of CARDs, a helicase domain and a C-terminal domain (CTD). CARDs are responsible for signal activation, while helicase and CTD are for RNA binding. The downstream adaptor MAVS contains the N-terminal CARD, followed by ~400 residue-long linker containing TRAF binding sites and a C-terminal transmembrane domain (TM). MAVS CARD interacts with RLR CARDs, while TM anchors MAVS to mitochondria. B) RIG-I and MDA5 both form filaments on dsRNA, but their assembly mechanisms differ. RIG-I first binds the 5’ppp at the end of the dsRNA, then uses ATP hydrolysis to translocate to the interior. MDA5 binds the interior of the dsRNA then cooperatively forms a filament in a ATP-independent manner towards the end of the dsRNA. C) RIPLET uses bivalency to selectively bind RIG-I filaments (intra-filament binding). However, on long RIG-I filaments, RIPLET can induce higher order oligomerization by cross-bridging RIG-I filaments (inter-filament binding). This results in clustering of RIG-I filaments and further amplification of antiviral signaling. D) A model of how filament formation and higher order oligomerization of RIG-I and MAVS enable self vs. non-self discrimination and antiviral signaling. RIG-I can bind short dsRNA as a monomer, but RNA-binding is insufficient to activate downstream signaling. Filament formation along long dsRNA allows recruitment of RIPLET, which in turn bridges RIG-I filaments and conjugates K63-linked Ub chains. The high local receptor concentration and K63-polyUb together promote RIG-I CARD tetramer formation, which then acts as a nucleus to induce MAVS filament formation. MAVS filament then functions as a signaling platform to recruit and activate further downstream signaling molecules, such as TRAFs. The multiple steps of receptor oligomerization serve as independent check-points to filter out self RNAs and allow only non-self RNAs to activate downstream signaling. Once MAVS filament is nucleated, signal amplifies through polymerization of MAVS. Thus, the combination of multi-step oligomerization, both by the receptor and the signaling adaptor, ensures the accuracy and robustness of antiviral signaling in response to infection.