Fig. 2. Selective expansion of the tumor-reactive TCR Vβ family in mice responsive to ACT.
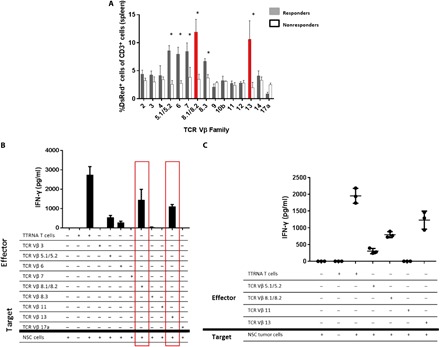
To generate antitumor T cells, total RNA is extracted from tumor cells and electroporated into syngeneic bone marrow–derived DCs. These cells are then cocultured with splenocytes from a previously immunized mouse with interleukin-2 for 5 to 7 days generating a polyclonal population of CD8+ T cells. After this ex vivo activation, 107 T cells are adoptively transferred into tumor-bearing mice followed by vaccination with 2.5 × 105 RNA-pulsed DCs. In the preclinical model of ACT, C57BL/6 mice receive orthotopic tumor followed by host conditioning with total body irradiation and hematopoietic stem cell transfer to protect from bone marrow failure. (A) Mice implanted with cerebellar NSC medulloblastoma were treated with ACT using DsRed+ tumor–reactive T cells. Spleens were harvested from all mice, and relative abundance of each TCR Vβ family was measured in both responders and nonresponders. Here, 25 mice are implanted with tumor and treated with ACT. The first five nonresponders that succumb to tumor are taken at humane end point and spleens were analyzed. The five responders are treated mice that demonstrate no evidence of tumor after 120 days. This experiment was repeated twice with the same results as shown. n = 5 to 7 mice per group. (B) Spleens of five asymptomatic long-term survivors were harvested at 90 days after ACT. DsRed+ T cells were isolated and separated by the TCR Vβ family. Each TCR Vβ family was cocultured in vitro against tumor cells, and IFN-γ secretion was measured. (C) Splenic T lymphocytes were harvested from nonresponders to therapy upon detection of tumor via bioluminescent imaging. DsRed+ T cells were FACS-isolated and sorted into TCR Vβ families and then used as effectors against the primary NSC cell line. IFN-γ was measured to determine antitumor reactivity.
