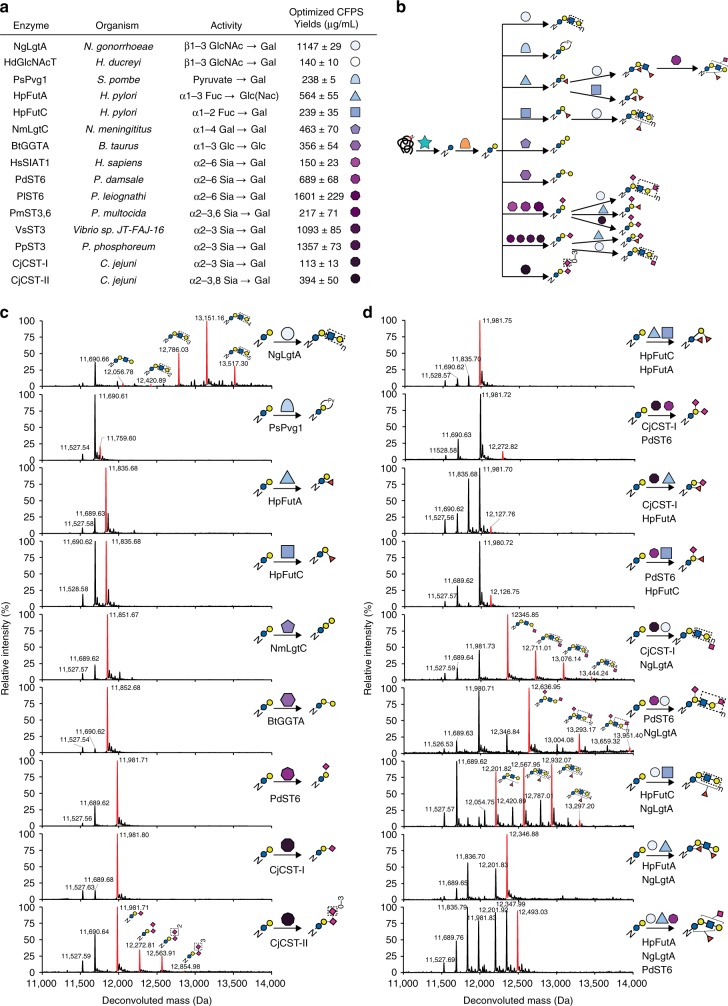
Fig. 3.
In vitro synthesis and assembly of complex glycosylation pathways. a Protein name, species, previously characterized specificity (Supplementary Table 4), and optimized CFPS soluble yields (Supplementary Table 2) for enzymes tested for elaboration of N-linked lactose. CFPS yields indicate mean and s.d. from n = 3 CFPS reactions quantified by [14C]-leucine incorporation. CjCST-I and HsSIAT1 yields were measured under oxidizing conditions (see Supplementary Fig. 9). (b) Intact deconvoluted MS spectra from Im7-6 protein purified from IVG reactions with 10 µM Im7-6, 0.4 µM ApNGT, 2 µM NmLgtB, and 2.5 mM appropriate nucleotide-activated sugar donors as well as 4.0 µM BtGGTA, 5.3 µM NmLgtC, 4.9 µM HpFutA, 2.6 µM HpFutC, 4.9 µM PdST6, 5.0 µM CjCST-II, 1.3 µM CjCST-I, 11.5 µM NgLgtA, or 2.2 µM SpPvg1. Mass shifts of intact Im7-6, fragmentation spectra of trypsinized Im7-6 glycopeptides (Supplementary Fig. 7), and exoglycosidase digestions (Supplementary Figs. 10-11) are consistent with modification of N-linked lactose with α1-3 Gal, α1-4 Gal, α1-3 Fuc, α2-6 Sia, α2-3 Sia, α2-8 Sia, β1-3 GlcNAc, or pyruvylation according to known activities of BtGGTA, NmLgtC, HpFutA, HpFutC, PdST6, CjCST-II, CjCST-I, NgLgtA, or SpPvg1. d Deconvoluted intact Im7-6 spectra of fucosylated and sialylated LacNAc structures produced by four- and five- enzyme combinations. IVG reactions contained 10 µM Im7-6, 0.4 µM ApNGT, 2 µM NmLgtB, appropriate sugar donors, and indicated GTs at half or one third the concentrations indicated in (b) for four- and five- enzyme pathways, respectively. Intact mass shifts and fragmentation spectra (Supplementary Fig. 12) are consistent with fucosylation and sialylation of the LacNAc core according to known activities. Intact protein and glycopeptide fragmentation spectra from other screened GTs and GT combinations not shown here are found in Supplementary Figs. 6–8 and 12–14. To provide maximum conversion, IVG reactions were incubated for 24 h at 30 °C, supplemented with an additional 2.5 mM sugar donors and incubated for another 24 h at 30 °C. Spectra were acquired from full elution areas of all detected glycosylated and aglycosylated Im7 species and are representative of n = 2 IVGs. Spectra from m/z 100–2000 were deconvoluted into 11,000–14,000 Da using Bruker Compass Data Analysis maximum entropy method. Source data is available in the Source Data file