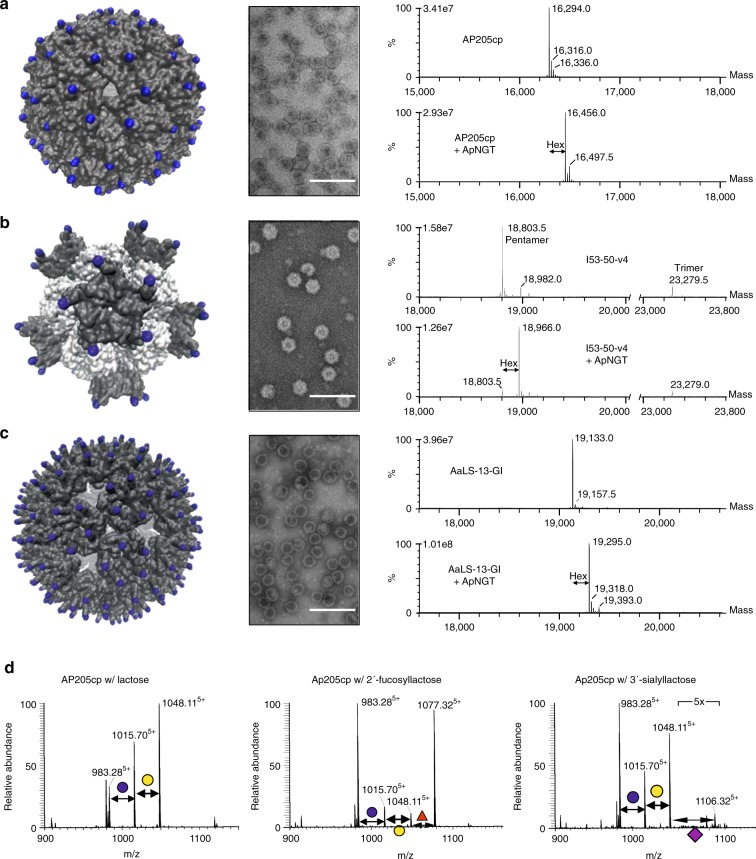
Fig. 3.
Glycosylation of megadalton protein assemblies. Glycosylation sites were introduced into surface-exposed regions of three self-assembling protein nano-structures. Each construct was expressed in the absence and presence of the NGT, particles were purified, and characterized. Glycosylation sites are indicated as blue spheres on the molecular model of each particle; a AP205 VLP, b I53-50-v4, trimer and pentamer subunits are white and gray, respectively, and c AaLS-13. Assembly of particles was assessed by transmission electron microscopy (scale bars represent 100 nm). Glucosylation was assessed by intact protein mass spectrometry, deconvoluted spectra are shown in the right panel. d The AP205 VLP was co-expressed with established pathways for lactose, 2′-fucosyllactose, and 3′-sialyllactose. Modification of the coat protein (AP205cp) was assessed by nano-LC–MS/MS analysis of tryptic peptides. MS spectra are the sum of all modified and unmodified peptides, in the 5+ charge state, with sequence TLFASGNAGLGFLDPTAAIVSSDTTAGSGGAHATANATAHATWSHPQFEK (where N is the potential N-glycosylation site). The assigned structures are supported by respective HCD–MS/MS spectra (Supplementary Fig. 10).