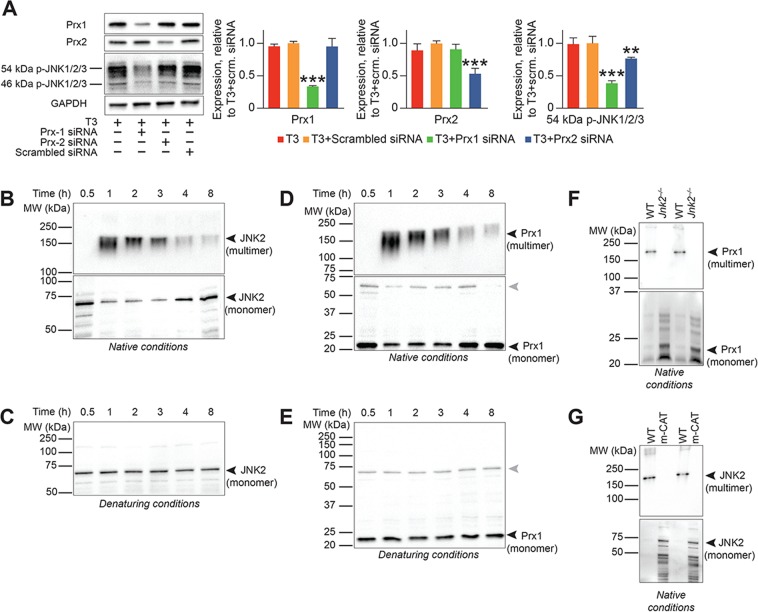
Figure 6.
The H2O2 sensor Prx1 plays a major role in T3-dependent JNK2 phosphorylation. (A) Representative immunoblot of lysates from cultured neonatal cardiomyocytes showing that knockdown of Prx1 and not Prx2 mainly prevents T3-dependent high molecular weight (54 kDa isoform) JNK phosphorylation. Representative of 4 biological replicates. Error bars indicate SEM. **P < 0.01; ***P < 0.001. Quantitative analyses of immunoblots is also shown. (B) Immunoblot of lysates under non-reducing conditions, to prevent dissociation of disulfide bridges, shows that T3-treatment results in a JNK2 multimer of ~160 kDa within 1 h with a concomitant decrease in the abundance of JNK2 monomer. The multimer dissociates over the next 3–4 h with the abundance of the ~60 kDa monomer increasing concomitantly. (C) Under reducing SDS-PAGE condition JNK2 fractionates as a single ~60 kDa species at all times. (D) Immunoblotting with Prx1-specific antibody under non-reducing conditions shows that T3-treatment results in a Prx1 multimer of ~160 kDa within 1 h, with a concomitant decrease in Prx1 monomer. The multimer dissociates over the next 2–3 h, which is associated with the concomitant detection of an ~22 kDa monomeric Prx1 species. (E) Immunoblotting T3-treated cardiomyocyte lysates under reducing SDS-PAGE condition shows that Prx1 fractionates as a single ~22 kDa species at all times. (F,G) In vivo, 1-h T3 treatment induces Prx1/JNK2 multimer formation (~160 kDa) in WT mice but not in Jnk2−/− (F) or m-CAT transgenic mice (G). Immunoblots in (B–G) are representative of 2–3 biological replicates.