Figure 4.
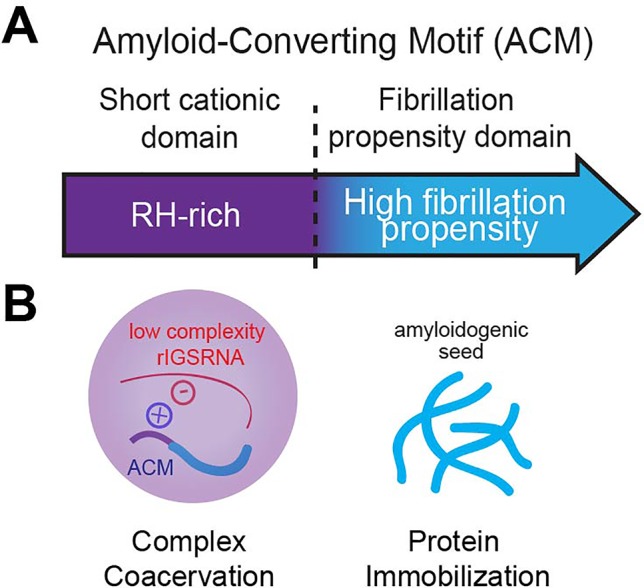
The amyloid-converting motif (ACM). (A) The ACM is necessary and sufficient to target and immobilize proteins in amyloid bodies (A-bodies). It consists of a R/H-rich short cationic domain flanking a high fibrillation propensity domain (determined by Rosetta energy of less than −23 kcal/mol). (B) We propose that it is its bipartite nature that allows the ACM to traverse phase boundaries. Complex coacervation of short cationic domains and low-complexity rIGSRNA (liquid–liquid phase separation) concentrates fibrillation propensity domains to activate a liquid-to-solid phase transition to form A-bodies.
