Abstract
BACKGROUND
Because of the powerful abilities of self-learning and handling complex biological information, artificial neural network (ANN) models have been widely applied to disease diagnosis, imaging analysis, and prognosis prediction. However, there has been no trained preoperative ANN (preope-ANN) model to preoperatively predict the prognosis of patients with gastric cancer (GC).
AIM
To establish a neural network model that can predict long-term survival of GC patients before surgery to evaluate the tumor condition before the operation.
METHODS
The clinicopathological data of 1608 GC patients treated from January 2011 to April 2015 at the Department of Gastric Surgery, Fujian Medical University Union Hospital were analyzed retrospectively. The patients were randomly divided into a training set (70%) for establishing a preope-ANN model and a testing set (30%). The prognostic evaluation ability of the preope-ANN model was compared with that of the American Joint Commission on Cancer (8th edition) clinical TNM (cTNM) and pathological TNM (pTNM) staging through the receiver operating characteristic curve, Akaike information criterion index, Harrell's C index, and likelihood ratio chi-square.
RESULTS
We used the variables that were statistically significant factors for the 3-year overall survival as input-layer variables to develop a preope-ANN in the training set. The survival curves within each score of the preope-ANN had good discrimination (P < 0.05). Comparing the preope-ANN model, cTNM, and pTNM in both the training and testing sets, the preope-ANN model was superior to cTNM in predictive discrimination (C index), predictive homogeneity (likelihood ratio chi-square), and prediction accuracy (area under the curve). The prediction efficiency of the preope-ANN model is similar to that of pTNM.
CONCLUSION
The preope-ANN model can accurately predict the long-term survival of GC patients, and its predictive efficiency is not inferior to that of pTNM stage.
Keywords: Gastric cancer, Artificial neural network model, Prognostic model, Preoperative, Blood biomarkers, Long-term survival
Core tip: We established an artificial neural network model before surgery that can predict the long-term survival of patients with gastric cancer, and its predictive efficiency is not inferior to that of pathological TNM stage.
INTRODUCTION
Gastric cancer (GC) is one of the five most common malignant tumors in the world, and it is the third leading cause of death in cancer patients[1]. Despite the progress in the diagnosis and treatment of GC, the prognosis of GC patients is still very poor, especially the long-term survival of advanced GC patients, for which the 5-year survival rate is only 10%[2]. Most scholars have focused on how to accurately distinguish the stages of GC in patients. The improved postoperative American Joint Commission on Cancer (AJCC) TNM staging system based on pathological examination is currently the most important and recognized prognostic staging system for GC[3-5]. However, this scoring system needs to be performed based on the pathological analysis of tumor specimens and examination of lymph nodes after surgery, which cannot provide a reference for preoperative treatment and consultation. Recently, the treatment for GC has gradually changed from simple surgical treatment to comprehensive treatment with the core being surgical treatment. Accurate preoperative tumor assessment providing a reasonable individualized treatment for GC patients is the key to improving the prognosis of patients with GC. However, the single traditional index, namely, the clinical TNM (cTNM) staging system based on the imaging examination, does not show the ideal accuracy of preoperative tumor assessment. Therefore, it has been a challenge in clinical work to explore the markers and methods for preoperative accurate tumor assessment.
In recent years, increasingly more scholars have believed that the inflammation indexes are relevant to the survival of cancer patients[6,7]. Some scholars have reported that inflammatory cells may promote tumor growth and progression of the disease, rather than produce an effective host antitumor response. Consequently, the inflammatory biomarkers in peripheral blood are potential predictors of cancer prognosis[8-11]. It has been reported that several indexes from peripheral blood, such as the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and albumin-globulin ratio (AGR), have a powerful impact on the prognosis of GC[8,11-14]. The prognostic nutrition index (PNI) based on lymphocyte and albumin levels is a feasible parameter to reflect the immune and nutritional status of patients with a malignant tumor and is regarded as a good index to evaluate the prognosis of patients with GC[3,15]. Although the previously established inflammatory models could predict the prognosis of patients with GC, their accuracy was still not satisfactory. Furthermore, many pathophysiological processes of malignant tumors are nonlinear processes, which cannot be well reflected through traditional linear analysis methods. Many studies have shown that artificial neural networks (ANNs) can deal with the nonlinear statistical relationship better than the traditional analysis methods, including studies on the prognosis of various cancers[16-18]. It has been reported that ANNs had a more accurate prognostic ability than TNM staging in patients with breast and rectal cancers[19]. However, there is no study on the relationship between a preoperative ANN (preope-ANN) and the prognosis in GC patients. Therefore, this study combined the preoperative blood biomarkers and preoperative tumor data to establish an ANN model in order to build a reliable preoperative prediction system that can achieve the same effect of postoperative TNM staging. The aim of this study was to evaluate the prognosis of patients with GC and to provide a reasonable individualized treatment plan for patients.
MATERIALS AND METHODS
Patients
The clinicopathological data of 1860 GC patients treated at the Department of Gastric Surgery, Fujian Medical University Union Hospital from January 2011 to April 2015 were analyzed retrospectively. The inclusion criteria were that all patients were diagnosed with GC and accepted radical surgery at our center. The exclusion criteria were as follows: (1) The general clinical data were incomplete, including age, sex, body mass index (BMI), American society of anesthesiologists (ASA) score, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA199), alpha fetoprotein (AFP), NLR, PLR, AGR, PNI, preoperative complications, tumor size, primary site, clinical T stage (cT), and clinical N stage (cN); (2) The follow-up data were missing; and (3) Patients receiving neoadjuvant chemotherapy. A total of 1608 patients were included (Supplementary Figure 1). The study was approved by the Ethics Committee of Fujian Medical University Union Hospital.
Data
All patients underwent routine preoperative examinations, including upper gastrointestinal endoscopy and upper gastrointestinal angiography, to assess tumor location. Preoperative clinical staging was assessed using chest radiography, total abdominal computed tomography (CT), abdominal ultrasound, and, if necessary, PET-CT, and bone scans. The 8th edition of the AJCC tumor staging system was used for cTNM and pathological TNM (pTNM) staging (Supplementary Figure 1). The overall survival (OS) time was the time from the operation to the last follow-up time or death.
All patients were randomly divided into a training set (n = 1104, 70%) and a testing set (n = 504, 30%), and the testing set was separated for evaluation of the final model. The training data were used five times for cross-validation to optimize the neural network. Finally, the separated data of the testing set were used to evaluate the model.
Blood sample analysis
Routine blood biochemistry and tumor markers were assessed within 1 wk before the operation. Using X-tile software, the optimal critical values of the NLR for OS were 1.91 and 3.87 (P < 0.05). According to optimal critical values, the patients were divided into three groups as follows: Low NLR group (NLR ≤ 1.91, n = 632), middle NLR group (1.91 < NLR ≤ 3.87, n = 753), and high NLR group (NLR > 3.87, n = 223). Similarly, the patients were divided into a low PLR group (PLR ≤ 89.5, n = 258), middle PLR group (89.5 < PLR ≤ 162.3, n = 777), and high PLR group (PLR > 162.3, n = 573). According to the AGR, the patients were divided into a low AGR group (AGR ≤ 6.59, n = 474), middle AGR group (6.59 < AGR ≤ 7.81, n = 612), and high AGR group (AGR > 7.81, n = 522). Furthermore, according to the PNI, the patients were divided into a low PNI group (PNI ≤ 371.01, n = 495), middle PNI group (371.0 < PNI ≤ 430.0, n = 771), and high PNI group (PNI > 430.0, n = 342).
Preope-ANN model
First, logistic univariate analysis was used to analyze 1104 cases in the training set to screen the variables that affected the 3-year survival and to determine these variable items as the input nodes of the ANN.
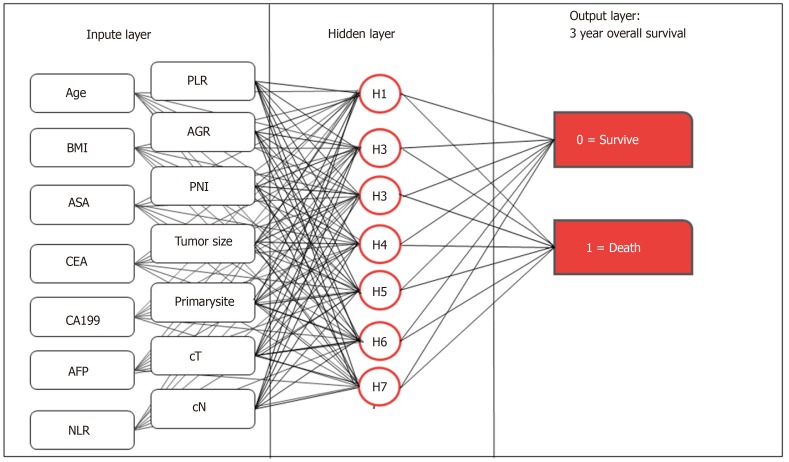
We used the multilayer perceptron to develop the preope-ANN (Figure 1). The input layer of the preope-ANN model consisted of the screened general clinicopathological data and preoperative blood biomarkers using logistic analysis and the 3-year survival condition. The ANN intelligently analyzed the 3-year survival of the patients according to the input data and generated the outcome compared with the real value. The output layer consisted of two values: (1) The patient's 3-year survival (death or survival); and (2) The continuous variables from 0 to 1, which represent the predictive probability of the outcome of the patient's 3-year survival. The network architecture of the preope-ANN model was composed of seven hidden layers, and the input layer was composed of 16 nodes (including 14 general clinical data and blood biomarkers and two survival conditions related to the 3-year OS). The hidden layer was activated by the hyperbolic tangent function, and the output layer was composed of two nodes activated by the softmax function, including the survival condition and survival probability. According to the survival probability predicted by the preope-ANN model, the patients were divided into seven subgroups: The survival probability was 0%-30% for group A, 30%-50% for group B, 50%-65% for group C, 65%-80% for group D, 80%-90% for group E, 90%-97% for group F, and 97%-100% for group G.
Figure 1.
Structure diagram of the preoperative artificial neural network model. Age, sex, body mass index, American society of anesthesiologists score, tumor location, tumor size, prognostic nutrition index, albumin-globulin ratio, neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, clinical T stage, clinical N stage, carcinoembryonic antigen, carbohydrate antigen 19-9, and alpha fetoprotein are the input variables. All weighted values passed to the hidden layer node are summed on the hidden layer node and passed to the output node through the sigmoid function. All weighted values entering the output node are summed again and passed through the sigmoid function. For each patient, the probability of output is 0-1.0. In the training of the artificial neural network, the output values are compared with the real results of each patient. The weight is adjusted so that the next time the patient appears on the network, the network output is closer to the real result. NLR: Neutrophil-lymphocyte ratio; PLR: Platelet-lymphocyte ratio; AGR: Albumin-globulin ratio; PNI: Prognostic nutrition index; BMI: Body mass index; ASA: American society of anesthesiologists score; cT: Clinical T stage; cN: Clinical N stage; CEA: Carcinoembryonic antigen; CA199: Carbohydrate antigen 19-9; AFP: Alpha fetoprotein.
Statistical analysis
All data were analyzed using SPSS 20.0 (SPSS Inc. Chicago, IL, United States) and R software (version 3.5.4). The classified variables were tested using the χ2 test or Fisher's exact test. The X-tile software was used to determine the best cut-off point of the counting data. Mann-Whitney U test was used to test the receiver operating characteristic (ROC) curve and the area under the curve (AUC), and the Z test was used to compare the AUCs (MedCal software). We used Harrell’s C index to measure the discriminatory ability of the different models[4,20]. The likelihood ratio chi-square was calculated by Cox regression to measure homogeneity; a higher score means better homogeneity[21]. The Akaike information criterion (AIC) within the Cox regression model was used to compare performances between two prognostic models; smaller AIC values represent better optimistic prognostic stratification[22]. We calculated the relative likelihood of two models using the following formula: Exp {[AIC (model A) - AIC (model B)]/2}. The relative likelihood represents the probability that model A minimizes information as effectively as model B and could thus be interpreted as a P value for the comparison of both AIC values[23]. P < 0.05 was considered statistically significant.
RESULTS
General information
Table 1 shows the clinical data of 1608 patients with GC. Among them, 1104 cases were in the training set, and 504 cases were in the testing set. The average age of all patients diagnosed was 60.72 years (range, 12-101 years), and the male to female ratio was 2.84:1. The proportion of patients diagnosed with stages I, II, III, and IVA disease in the cTNM system was 11.6%, 38.2%, 45.6%, and 4.6%, respectively. The proportion of patients diagnosed with stage I, II, and III disease in the pTNM system was 22.6%, 24.9%, and 52.5%, respectively.
Table 1.
General clinicopathological data, n (%)
| Variable | All patients (n = 1608) | Training set (n = 1104) | Testing set (n = 504) |
| Age (yr) | |||
| < 57 | 593 (36.9) | 413 (37.4) | 180 (35.7) |
| 57-74 | 838 (52.1) | 565 (51.2) | 273 (54.2) |
| > 74 | 177 (11.0) | 126 (11.4) | 51 (10.1) |
| Sex | |||
| Female | 412 (25.6) | 299 (27.1) | 113 (22.4) |
| Male | 1196 (74.4) | 805 (72.9) | 391 (77.6) |
| BMI | |||
| < 18.5 | 156 (9.7) | 98 (8.9) | 58 (11.5) |
| 18.5-23.5 | 960 (59.7) | 670 (60.7) | 290 (57.5) |
| > 23.5 | 492 (30.6) | 336 (30.4) | 156 (31.0) |
| CEA | |||
| < 2.8 | 932 (58.0) | 626 (56.7) | 306 (60.7) |
| 2.8-4.8 | 328 (20.4) | 226 (20.5) | 102 (20.2) |
| > 4.8 | 348 (21.6) | 252 (22.8) | 96 (19.0) |
| CA199 | |||
| < 15.0 | 1043 (64.9) | 696 (63.0) | 347 (68.8) |
| 15.0-39.2 | 348 (21.6) | 255 (23.1) | 93 (18.5) |
| > 39.2 | 217 (13.5) | 153 (13.9) | 64 (12.7) |
| AFP | |||
| < 2 | 496 (30.8) | 344 (31.2) | 152 (30.2) |
| 2-5.29 | 916 (57.0) | 631 (57.2) | 285 (56.5) |
| > 5.3 | 196 (12.2) | 129 (11.7) | 67 (13.3) |
| NLR | |||
| < 1.91 | 632 (39.3) | 422 (38.2) | 210 (41.7) |
| 1.91-3.87 | 753 (46.8) | 525 (47.6) | 228 (45.2) |
| > 3.87 | 223 (13.9) | 157 (14.2) | 66 (13.1) |
| PLR | |||
| < 89.5 | 258 (16.0) | 177 (16.0) | 81 (16.1) |
| 89.5-162.3 | 777 (48.3) | 540 (48.9) | 237 (47.0) |
| >162.3 | 573 (35.6) | 387 (35.1) | 186 (36.9) |
| AGR | |||
| < 6.59 | 474 (29.5) | 332 (30.1) | 142 (28.2) |
| 6.59-7.81 | 612 (38.1) | 426 (38.6) | 186 (36.9) |
| > 7.81 | 522 (32.5) | 346 (31.3) | 176 (34.9) |
| PNI | |||
| < 371 | 495 (30.8) | 347 (31.4) | 148 (29.4) |
| 371-430 | 771 (47.9) | 531 (48.1) | 240 (47.6) |
| > 430 | 342 (21.3) | 226 (20.5) | 116 (23.0) |
| ASA | |||
| I | 983 (61.1) | 677 (61.3) | 306 (60.7) |
| II | 561 (34.9) | 379 (34.3) | 182 (36.1) |
| III-IV | 64 (4.0) | 48 (4.3) | 16 (3.2) |
| Comorbidity | |||
| No | 1133 (70.5) | 779 (70.6) | 354 (70.2) |
| Yes | 475 (29.5) | 325 (29.4) | 150 (29.8) |
| Primary site | |||
| Lower | 671 (41.7) | 476 (43.1) | 195 (38.7) |
| Middle | 350 (21.8) | 231 (20.9) | 119 (23.6) |
| Upper | 401 (24.9) | 257 (23.3) | 144 (28.6) |
| Overlapping | 186 (11.6) | 140 (12.7) | 46 (9.1) |
| Tumor size (mm) | |||
| < 30 | 578 (35.9) | 404 (36.6) | 174 (34.5) |
| 30-60 | 711 (44.2) | 477 (43.2) | 234 (46.4) |
| > 60 | 319 (19.8) | 223 (20.2) | 96 (19.0) |
| cT | |||
| T1 | 167 (10.4) | 108 (9.8) | 59 (11.7) |
| T2 | 185 (11.5) | 135 (12.2) | 50 (9.9) |
| T3 | 429 (26.7) | 292 (26.4) | 137 (27.2) |
| T4 | 827 (51.4) | 569 (51.5) | 258 (51.2) |
| cN | |||
| N0 | 662 (41.2) | 457 (41.4) | 205 (40.7) |
| N1 | 376 (23.4) | 257 (23.3) | 119 (23.6) |
| N2 | 362 (22.5) | 245 (22.2) | 117 (23.2) |
| N3 | 208 (12.9) | 145 (13.1) | 63 (12.5) |
| Follow-up duration (mo) | 48 (3-91) | 48 (3-91) | 50 (3-89) |
BMI: Body mass index; CEA: Carcinoembryonic antigen; CA199: Carbohydrate antigen 19-9; AFP: Alpha fetoprotein; NLR: Neutrophil-lymphocyte ratio; PLR: Platelet-lymphocyte ratio; AGR: Albumin-globulin ratio; PNI: Prognostic nutrition index; ASA: American society of anesthesiologists; cT: Clinical T stage; cN: Clinical N stage.
Univariate logistic analysis of the survival of patients in the training set
In the training set, the univariate logistics regression analysis (Table 2) showed that age, sex, BMI, CEA, CA199, AFP, NLR, PLR, AGR, PNI, ASA score, tumor location, tumor size, cT stage, and cN stage were significant factors for the 3-year OS of the patients (P < 0.05 for all).
Table 2.
Univariate logistics regression analysis of risk factors for the 3-year survival
| Variable |
Training set |
|||
| OR | 95CI | P value | ||
| Age (yr) | ||||
| < 57 | REF | |||
| 57-74 | 0.38 | 0.25 | 0.58 | < 0.001 |
| > 74 | 0.63 | 0.42 | 0.93 | 0.020 |
| Sex | ||||
| Male | REF | |||
| Female | 0.96 | 0.72 | 1.29 | 0.782 |
| BMI | ||||
| < 18.5 | REF | |||
| 18.5-23.5 | 3.04 | 1.90 | 4.85 | < 0.001 |
| > 23.5 | 1.28 357 | 0.95 | 1.73 | 0.111 |
| CEA | ||||
| < 2.8 | REF | |||
| 2.8-4.8 | 0.33 | 0.24 | 0.45 | < 0.001 |
| > 4.8 | 0.46 | 0.31 | 0.67 | < 0.001 |
| CA199 | ||||
| < 15.0 | REF | |||
| 15.0-39.2 | 0.20 | 0.14 | 0.29 | < 0.001 |
| > 39.2 | 0.33 | 0.22 | 0.50 | < 0.001 |
| AFP | ||||
| < 2 | REF | < 0.001 | ||
| 2-5.29 | 0.78 | 0.51 | 1.18 | 0.240 |
| > 5.3 | 0.50 | 0.33 | 0.74 | 0.001 |
| NLR | ||||
| < 1.91 | REF | |||
| 1.91-3.87 | 0.32 | 0.22 | 0.48 | < 0.001 |
| > 3.87 | 0.48 | 0.33 | 0.69 | < 0.001 |
| PLR | ||||
| < 89.5 | REF | |||
| 89.5-162.3 | 0.26 | 0.16 | 0.42 | < 0.001 |
| > 162.3 | 0.59 | 0.45 | 0.78 | < 0.001 |
| AGR | ||||
| < 6.59 | REF | |||
| 6.59-7.81 | 2.11 | 1.50 | 2.98 | < 0.001 |
| > 7.81 | 1.58 | 1.13 | 2.20 | 0.007 |
| PNI | ||||
| < 371 | REF | |||
| 371-430 | 3.98 | 2.62 | 6.06 | < 0.001 |
| > 430 | 1.94 | 1.29 | 2.92 | 0.002 |
| ASA | ||||
| I | REF | |||
| II | 0.44 | 0.24 | 0.79 | 0.006 |
| III-IV | 0.53 | 0.29 | 0.97 | 0.041 |
| Comorbidity | ||||
| No | REF | |||
| Yes | 0.99 | 0.74 | 1.31 | 0.933 |
| Primary site | ||||
| Lower | REF | |||
| Middle | 0.30 | 0.20 | 0.45 | < 0.001 |
| Upper | 0.54 | 0.35 | 0.83 | 0.001 |
| Overlapping lesion of the stomach | 0.47 | 0.31 | 0.73 | < 0.001 |
| Tumor size (mm) | ||||
| < 30 | REF | |||
| 30-60 | 0.08 | 0.06 | 0.13 | < 0.001 |
| > 60 | 0.43 | 0.31 | 0.60 | < 0.001 |
| cT | ||||
| T1 | REF | |||
| T2 | 0.05 | 0.02 | 0.13 | < 0.001 |
| T3 | 0.18 | 0.11 | 0.32 | < 0.001 |
| T4 | 0.24 | 0.17 | 0.34 | < 0.001 |
| cN | ||||
| N0 | REF | |||
| N1 | 0.18 | 0.12 | 0.27 | < 0.001 |
| N2 | 0.28 | 0.18 | 0.43 | < 0.001 |
| N3 | 0.67 | 0.44 | 1.007 | 0.054 |
BMI: Body mass index; CEA: Carcinoembryonic antigen; CA199: Carbohydrate antigen 19-9; AFP: Alpha fetoprotein; NLR: Neutrophil-lymphocyte ratio; PLR: Platelet-lymphocyte ratio; AGR: Albumin-globulin ratio; PNI: Prognostic nutrition index; ASA: American society of anesthesiologists; cT: Clinical T stage; cN: Clinical N stage; OR: Odds ratio; CI: Confidence interval.
Performance evaluation of the preope-ANN model
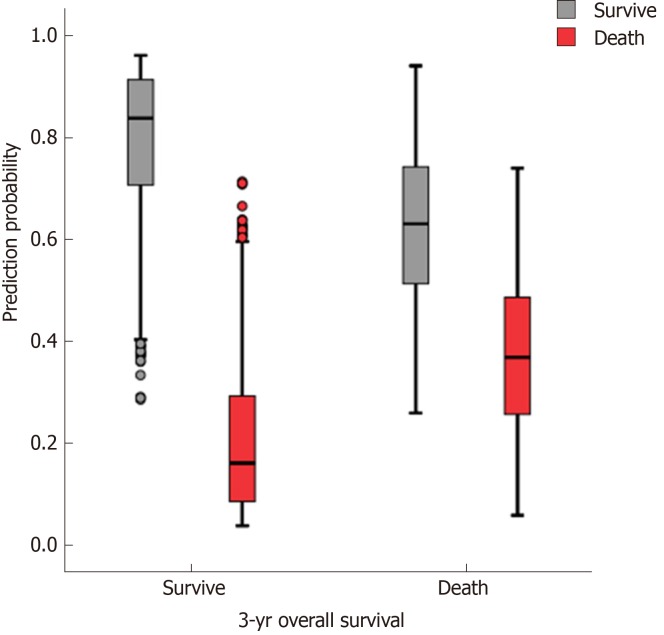
The accuracy, sensitivity, and specificity of the preope-ANN model in the training set were 77.3%, 88.5%, and 50.1%, respectively. For the preope-ANN model in the testing set, the accuracy was 75.2%, the sensitivity was 86.5%, and the specificity was 43.8% (Figure 2).
Figure 2.
Prediction of the 3-year survival of patients using the preoperative artificial neural network. In the training set, the predictive accuracy rate was 77.3%. The sensitivity and specificity of the centralized training model were 88.5% and 50.1%, respectively. The accuracy of the testing set model for survival prediction was 75.2%. The sensitivity and specificity of the test set model were 86.5% and 43.1%, respectively.
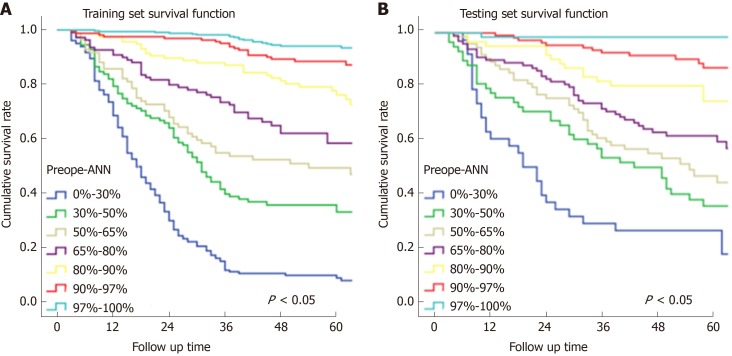
Subgroups of the preope-ANN model for Kaplan-Meier survival analysis
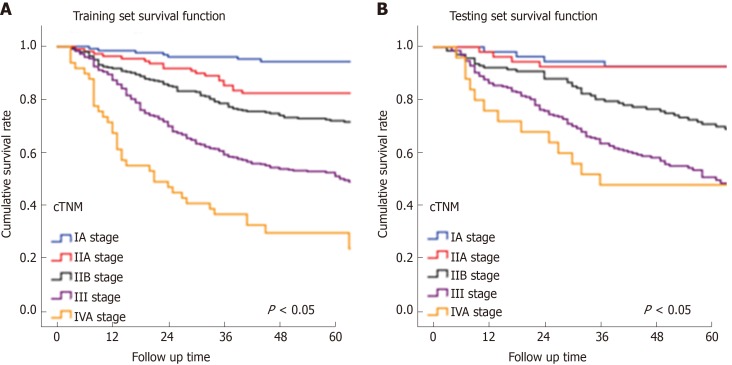
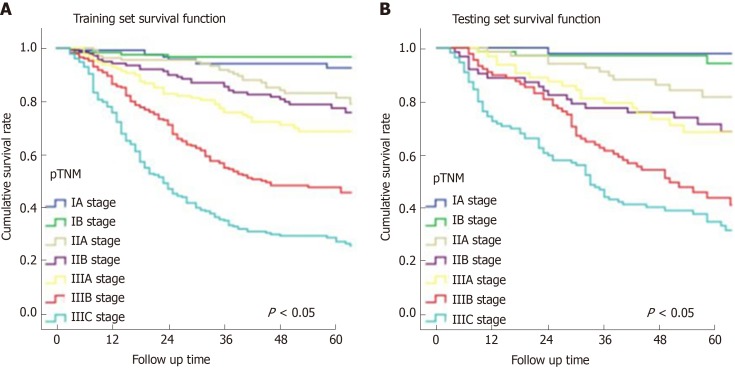
In Figure 3, the prediction results of the preope-ANN model were divided into seven subgroups. The Kaplan-Meier survival curve showed that the survival curve of each subgroup within the preope-ANN model had good discrimination in both the training and testing sets (P < 0.05). For cTNM staging, the survival curve in the training set showed (Figure 4) that the substages of the TNM system were well differentiated (P < 0.05). However, the survival curve of the testing set showed that the curve of stage III was close to that of stage IVA (P = 0.335). Figure 5 shows the survival analysis of the pTNM staging. In the training set, the survival curve discrimination between stages IA and IB was poor (P = 0.240), and there was no significant difference between stages IIA and IIB and stages IIB and IIIA (P < 0.05). In the testing set, there was no significant difference in survival curves between stages IA and IB, IIA and IIB, and IIIA and IIIB (P > 0.05).
Figure 3.
Subgroup survival curves of the preoperative artificial neural network. A: The survival curve of each subgroup of the preoperative artificial neural network model showed good discrimination in the training and testing sets (P < 0.05). B: The survival analysis of the training set showed that the substages of the TNM system were well differentiated (P < 0.05). Preope-ANN: Preoperative artificial neural network.
Figure 4.
Survival curves of the clinical TNM staging. A: The discrimination between the survival curves of each stage of the clinical TNM (cTNM) system in the training set was good (P < 0.05). B: There was no significant difference in the survival curves between the cTNM stages I and II A (P = 0.935), and between stages III and IVA (P = 0.355). cTNM: Clinical TNM.
Figure 5.
Survival curves of the pathological TNM staging. A: In the training set, the survival curve discrimination between stages IA and IB was poor (P = 0.240), and there was no significant difference in the survival curves between stages IIA and IIB and IIB and IIIA (P < 0.05). B: In the testing set, there was no significant difference in survival curves between stages IA and IB, IIA and IIB, and IIIA and IIIB of the pathological TNM staging (P > 0.05). pTNM: Pathological TNM.
Accuracy of each model
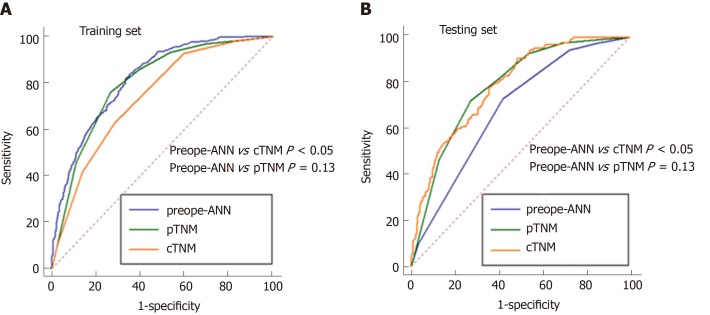
Figure 6 shows the ROC curves of the preope-ANN model, cTNM stage, and pTNM stage in both the training set and the testing set. In the training set, the AUC values of the preope-ANN model, cTNM stage, and pTNM stage were 0.820 (0.800-0.838), 0.740 (0.718-0.762), and 0.803 (0.782-0.822), respectively. The predictive performance of the preope-ANN was better than that of the cTNM stage (P < 0.05) and was similar to that of the pTNM stage (P = 0.130). In the testing set, the AUC values of the preope-ANN model, cTNM stage, and pTNM stage were 0.790 (0.752- 0.825), 0.687 (0.644-0.727), and 0.786 (0.748-0.821), respectively. Comparison of the AUC showed that the prediction performance of the preope-ANN was better than that of the cTNM stage (P < 0.05), and was similar to that of the pTNM (P = 0.858).
Figure 6.
Comparison of the receiver operating characteristic curves among the preope-ANN model, clinical TNM stage, and pathological TNM stage. A: In the training set, the area under the curve (AUC) values of the preoperative artificial neural network (preope-ANN) model, clinical TNM (cTNM) staging, and pathological TNM (pTNM) staging were 0.820 (0.800-0.838), 0.740 (0.718-0.762), and 0.803 (0.782-0.822), respectively. The comparison of the AUC values in the training set showed that the predictive performance of the preope-ANN was better than that of the cTNM stage (P < 0.05), and similar to that of pTNM stage (P = 0.130). B: In the testing set, the AUC values of the preope-ANN model, cTNM staging, and pTNM staging were 0.790 (0.752-0.825), 0.687 (0.644-0.727), and 0.786 (0.748-0.821), respectively. The comparison of the AUC values in the testing set showed that the prediction performance of the preope-ANN and the pTNM stage was better than that of the cTNM stage (P < 0.05), and the prediction performance of the preope-ANN was similar to that of the pTNM stage (P = 0.858). pTNM: Pathological TNM; cTNM: Clinical TNM; preope-ANN: Preoperative artificial neural network.
Comparison of the three models
As shown in Table 3, for Harrell's C index, the preope-ANN model was superior to the cTNM staging model and had the same performance as the pTNM staging model in both the training and testing sets. (In the training set: Preope-ANN vs cTNM = 0.773 vs 0.663, respectively, P < 0.001; preope-ANN vs pTNM = 0.773 vs 0.757, respectively, P = 0.120; in the testing set: Preope-ANN vs cTNM = 0.752 vs 0.652, respectively, P < 0.001; preope-ANN vs pTNM =0.752 vs 0.740, respectively, P = 0.539). The AIC analysis showed that the preope-ANN model of the training set had a better fitting degree than both the cTNM staging and pTNM staging (preope-ANN vs cTNM = 4977.83 vs 5176.70, respectively, relative likelihood < 0.001; preope-ANN vs pTNM = 4977.83 vs 4999.80, respectively, relative likelihood < 0.001). The fitting degree of the preope-ANN model in the testing set was better than that of the cTNM staging (preope-ANN vs cTNM = 1952.94 vs 2020.37, respectively, relative likelihood < 0.001), and the fitting degree of the preope-ANN model was not inferior to that of the pTNM staging (preope-ANN 1952.94 vs pTNM 1951.84, respectively, relative likelihood = 1.733). Therefore, the performance of the preope-ANN was better than that of the cTNM staging and was similar to that of the pTNM staging.
Table 3.
Comparison of the area under the curve values and Harrell’s C index between the pathological TNM stage, clinical TNM stage, and preoperative artificial neural network
|
Training set |
Testing set |
Bio-ANN | Cli-ANN | |||||
| Preope-ANN | cTNM | pTNM | Preope-ANN | cTNM | pTNM | |||
| Harrell’s C index | 0.773 (0.753-0.795) | 0.663 (0.640-0.687) | 0.757 (0.735-0.779) | 0.752 (0.719-0.785) | 0.652 (0.615-0.688) | 0.740 (0.707-0.775) | 0.722 (0.698-0.746) | 0.760 (0.738-0.782) |
| P value | < 0.001 | 0.120 | < 0.001 | 0.539 | aP < 0.001; bP = 0.000; cP = 0.018 | dP < 0.001 eP < 0.000; fP = 0.827 | ||
| AIC | 4977.83 | 5176.70 | 4999.80 | 1952.94 | 2020.37 | 1951.84 | 5115.9 | 5011.9 |
| Relative likelihood | < 0.001 | < 0.001 | <0.001 | 1.733 | aP < 0.001; bP > 1 cP < 0.001 | dP = 0.001 E > 1 fP = 0.06 | ||
P, Bio-artificial neural network (ANN) vs preoperative ANN (preope-ANN);
P, Bio-ANN vs clinical TNM (cTNM);
P, Bio-ANN vs pathological TNM (pTNM).
P, cli-ANN vs preope-ANN;
P, cli-ANN vs cTNM;
P, cli-ANN vs pTNM. pTNM: Pathological TNM; cTNM: Clinical TNM; preope-ANN: Preoperative artificial neural network; AIC: Akaike information criterion.
DISCUSSION
Presently, GC remains a common malignant tumor worldwide and the third leading cancer cause of death. As is known, it is very important to develop a GC prognostic model in order to provide valuable prognosis information for the patients and help clinicians formulate reasonable treatment regimens for the patients[19]. The TNM staging system proposed by the AJCC is the most important prognostic evaluation system for GC, and it has served as the main instruction for clinicians to choose the treatment plan. However, pTNM staging needs both grouping information and prognostic information from the postoperative histopathology results of the tumor specimen, which prevents the approach from guiding the preoperative treatment decision[24]. The exact pretreatment clinical stage is essential to customize the treatment strategy for each patient. Park et al[25] suggested that the clinical staging based on endoscopy and the CT scan has predictive value, where cTNM staging can be used to guide the treatment of GC patients; however, its accuracy depends on the imaging experience of the physician. Some scholars found that this method had limitations in evaluating the cT stage of large tumors, while the accuracy of cN was only 20%; and over 80% of pN0 patients are overestimated[26,27]. Thus, there is an urgent need for a more accurate preoperative prognostic model to guide the choice of treatment options.
In recent years, increasingly more studies have shown that blood inflammatory markers are associated with a poor prognosis in cancer patients[28]. The NLR, PLR, and PNI in cancer patients have been proven to be prognostic markers for various malignant tumors[29-32]. In our study, logistic analysis confirmed that the preoperative NLR, PLR, PNI, and AGR were significant prognostic factors for the 3-year survival, which is consistent with previous studies. However, because of the nonlinearity of biological information in the human body, the traditional model inevitably has had some limitations when the traditional linear analysis method was used to construct the prognostic model in previous studies. At the same time, the growth of a tumor is a process of interaction between the human body and the tumor, which depends on the nutritional status of the body, the immune system, and the tumor malignancy; thus, the application of a single index for forecasting may lack accuracy[18]. Consequently, we need a new statistical model that can synthesize the biological indicators and better address the nonlinear relationship among the indicators.
The ANN is a new computational model developed by simulating the function of human brain; this method can establish a nonlinear statistical model to evaluate complex biological systems and address the relationship between complex biological indicators more flexibly[33]. In recent years, ANNs have been successfully applied to the field for the identification of lesions in pathological specimens, automatic detection of breast X-ray injury, and disease diagnosis and treatment[33,34].
We synthesized preoperative blood biomarkers (the inflammatory indicators and PNI) and preoperative clinical data to establish the preope-ANN, which are easily available compared with the need for postoperative pathological results. At the same time, we used the ANN to reduce the error of human interference, ensuring the objectivity and accuracy of the results. The verification results showed that the accuracy of the preope-ANN model in predicting the 3-year survival rate was 91.7%. In addition, the comparison of Harrell's C index and AIC analysis showed that the accuracy and the fitting degree of the preope-ANN model were better than those of cTNM staging, and the preope-ANN model could achieve the same prediction effect as pTNM staging. The TNM staging system divides the patients into different risk groups, and our preope-ANN model can provide an even more detailed prediction for each patient, which is better than grouping the predictions. The preope-ANN model can be used to predict the long-term survival of patients before surgery and to choose a reasonable individualized treatment according to the prognosis. We can obtain the possible poor prognosis information of those patients with a low score before surgery and improve the prognosis by adopting neoadjuvant radiotherapy and chemotherapy.
This study still has some limitations. First, some patients had a follow-up period less than 5 years, and we only conducted the study for the 3-year survival outcome, not for the longer-term survival outcome. Second, this was a retrospective study, and some potential biases were still unavoidable. Moreover, this study included all postoperative patients, and the results are not suitable for the evaluation of the prognosis of patients with unresectable advanced GC. Nevertheless, this study first confirmed that the preope-ANN is a novel and convenient prognostic model through the use of a large sample data size, which can effectively predict the prognosis of GC patients. In the clinic, the preope-ANN model can be considered as part of preoperative risk stratification to guide the individualized treatment of patients with GC. The next challenge is to establish a web version of the preope-ANN model that can be dynamically adjusted for the input of different sample data; with this approach, the model accuracy would be closer to the real value and more flexibly applied to the evaluation of clinical patients.
ARTICLE HIGHLIGHTS
Research background
Because of the powerful abilities of self-learning and handling complex biological information, artificial neural network (ANN) models have been widely applied to disease diagnosis, imaging analysis, and prognosis prediction. However, there has been no trained preoperative ANN (preope-ANN) model to preoperatively predict the prognosis of patients with gastric cancer (GC).
Research motivation
This study combined the preoperative blood biomarkers and preoperative tumor data to establish an ANN model in order to build a reliable preoperative prediction system that can achieve the same effect as postoperative TNM staging. The aim of this study was to evaluate the prognosis of patients with GC and to provide a reasonable individualized treatment plan for patients.
Research objectives
We aimed to establish a neural network model that can predict long-term survival of GC patients before surgery to evaluate the tumor condition before the operation.
Research methods
The clinicopathological data of 1608 GC patients treated from January 2011 to April 2015 at the Department of Gastric Surgery, Fujian Medical University Union Hospital were analyzed retrospectively. Patients were randomly divided into a training set (70%) for establishing a preope-ANN model and a testing set (30%). The prognostic evaluation ability of the preope-ANN model was compared with that of the American Joint Commission on Cancer (8th edition) clinical TNM stage (cTNM) and pathological TNM stage (pTNM) through the receiver operating characteristic curve, Akaike information criterion index, Harrell's C index, and likelihood ratio chi-square.
Research results
We used the variables that were statistically significant factors for the 3-year overall survival as input-layer variables to develop a preope-ANN in the training set. The survival curves within each score of the preope-ANN had good discrimination (P < 0.05). Comparing the preope-ANN model, cTNM, and pTNM in both the training and testing sets, the preope-ANN model was superior to cTNM in predictive discrimination (C index), predictive homogeneity (likelihood ratio chi-square), and prediction accuracy (area under the curve). The prediction efficiency of the preope-ANN model was similar to that of pTNM.
Research conclusions
The preope-ANN model can accurately predict the long-term survival of GC patients, and its predictive efficiency is not inferior to pTNM staging.
Research perspectives
This study for the first time confirmed that the preope-ANN is a novel and convenient prognostic model through the use of a large sample data size, which can effectively predict the prognosis of GC patients. In the clinic, preope-ANN can be considered as part of preoperative risk stratification to guide the individualized treatment of patients with GC. The next challenge is to establish a web version of the preope-ANN model that can be dynamically adjusted for the input of different sample data; with this approach, the model accuracy would be closer to the real value and more flexibly applied to the evaluation of clinical patients.
Footnotes
Institutional review board statement: This retrospective study was approved by the Ethics Committee of Fujian Medical University Union Hospital.
Informed consent statement: The patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: All authors declare that they have no conflicts of interest.
Data sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Peer-review started: August 19, 2019
First decision: September 10, 2019
Article in press: October 17, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lazăr DC, de Melo FF, Nagem RG S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Ma YJ
Contributor Information
Si-Jin Que, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Qi-Yue Chen, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Qing-Zhong, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Zhi-Yu Liu, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Jia-Bin Wang, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Jian-Xian Lin, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Jun Lu, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Long-Long Cao, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Mi Lin, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Ru-Hong Tu, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Ze-Ning Huang, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Ju-Li Lin, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Hua-Long Zheng, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Ping Li, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Chao-Hui Zheng, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Chang-Ming Huang, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China.
Jian-Wei Xie, Department of Gastric Surgery and Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China; Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou 350108, Fujian Province, China; Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou 350108, Fujian Province, China. xjwhw2019@163.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Dassen AE, Dikken JL, van de Velde CJ, Wouters MW, Bosscha K, Lemmens VE. Changes in treatment patterns and their influence on long-term survival in patients with stages I-III gastric cancer in The Netherlands. Int J Cancer. 2013;133:1859–1866. doi: 10.1002/ijc.28192. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 5.Hirahara N, Tajima Y, Fujii Y, Yamamoto T, Hyakudomi R, Taniura T, Kaji S, Kawabata Y. Preoperative Prognostic Nutritional Index Predicts Long-term Outcome in Gastric Cancer: A Propensity Score-matched Analysis. Anticancer Res. 2018;38:4735–4746. doi: 10.21873/anticanres.12781. [DOI] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 9.Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, Sun H, Chen J, Wang F, Gao T, Zhang L, Wang S. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13:66. doi: 10.1186/s12967-015-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan QX, Su ZJ, Zhang JH, Wang CR, Ke SY. A comparison of the prognostic value of preoperative inflammation-based scores and TNM stage in patients with gastric cancer. Onco Targets Ther. 2015;8:1375–1385. doi: 10.2147/OTT.S82437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Wu Z, Lin E, Li W, Chen Y, Sun X, Zhou Z. Systemic prognostic score and nomogram based on inflammatory, nutritional and tumor markers predict cancer-specific survival in stage II-III gastric cancer patients with adjuvant chemotherapy. Clin Nutr. 2019;38:1853–1860. doi: 10.1016/j.clnu.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Cucchetti A, Piscaglia F, Grigioni AD, Ravaioli M, Cescon M, Zanello M, Grazi GL, Golfieri R, Grigioni WF, Pinna AD. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010;52:880–888. doi: 10.1016/j.jhep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 13.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, Lane S, Lee DY, Kaper M, McKean M, Beckermann KE, Rubinstein SM, Rooney I, Musib L, Budha N, Hsu J, Nowicki TS, Avila A, Haas T, Puligandla M, Lee S, Fang S, Wargo JA, Gershenwald JE, Lee JE, Hwu P, Chapman PB, Sosman JA, Schadendorf D, Grob JJ, Flaherty KT, Walker D, Yan Y, McKenna E, Legos JJ, Carlino MS, Ribas A, Kirkwood JM, Long GV, Johnson DB, Menzies AM, Davies MA. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–322. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Sun X, Liu J, Kong P, Chen S, Zhan Y, Xu D. Preoperative C-Reactive Protein/Albumin Ratio Predicts Prognosis of Patients after Curative Resection for Gastric Cancer. Transl Oncol. 2015;8:339–345. doi: 10.1016/j.tranon.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma M, Wang J, Hu Y, Weng M, Liu X, Wang Y. Prognostic Value of Inflammatory Biomarkers in Gastric Cancer Patients and the Construction of a Predictive Model. Dig Surg. 2019;36:433–442. doi: 10.1159/000493432. [DOI] [PubMed] [Google Scholar]
- 16.Burke HB, Goodman PH, Rosen DB, Henson DE, Weinstein JN, Harrell FE, Jr, Marks JR, Winchester DP, Bostwick DG. Artificial neural networks improve the accuracy of cancer survival prediction. Cancer. 1997;79:857–862. doi: 10.1002/(sici)1097-0142(19970215)79:4<857::aid-cncr24>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Lancashire LJ, Lemetre C, Ball GR. An introduction to artificial neural networks in bioinformatics--application to complex microarray and mass spectrometry datasets in cancer studies. Brief Bioinform. 2009;10:315–329. doi: 10.1093/bib/bbp012. [DOI] [PubMed] [Google Scholar]
- 18.Davies AR, Gossage JA, Zylstra J, Mattsson F, Lagergren J, Maisey N, Smyth EC, Cunningham D, Allum WH, Mason RC. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32:2983–2990. doi: 10.1200/JCO.2014.55.9070. [DOI] [PubMed] [Google Scholar]
- 19.Marrelli D, Morgagni P, de Manzoni G, Coniglio A, Marchet A, Saragoni L, Tiberio G, Roviello F Italian Research Group for Gastric Cancer (IRGGC) Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg. 2012;255:486–491. doi: 10.1097/SLA.0b013e3182389b1a. [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Yoon HM, Ryu KW, Nam BH, Cho SJ, Park SR, Lee JY, Lee JH, Kook MC, Choi IJ, Kim YW. Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg. 2012;214:88–96. doi: 10.1016/j.jamcollsurg.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) Psychol Methods. 2012;17:228–243. doi: 10.1037/a0027127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edeline J, Blanc JF, Johnson P, Campillo-Gimenez B, Ross P, Ma YT, King J, Hubner RA, Sumpter K, Darby S, Evans J, Iwuji C, Swinson D, Collins P, Patel K, Muazzam I, Palmer DH, Meyer T. A multicentre comparison between Child Pugh and Albumin-Bilirubin scores in patients treated with sorafenib for Hepatocellular Carcinoma. Liver Int. 2016;36:1821–1828. doi: 10.1111/liv.13170. [DOI] [PubMed] [Google Scholar]
- 24.Krittanawong C, Johnson KW, Rosenson RS, Wang Z, Aydar M, Baber U, Min JK, Tang WHW, Halperin JL, Narayan SM. Deep learning for cardiovascular medicine: a practical primer. Eur Heart J. 2019;40:2058–2073. doi: 10.1093/eurheartj/ehz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SR, Kim MJ, Ryu KW, Lee JH, Lee JS, Nam BH, Choi IJ, Kim YW. Prognostic value of preoperative clinical staging assessed by computed tomography in resectable gastric cancer patients: a viewpoint in the era of preoperative treatment. Ann Surg. 2010;251:428–435. doi: 10.1097/SLA.0b013e3181ca69a7. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu K, Ito K, Matsunaga N, Shimizu A, Kawakami Y. Diagnosis of gastric cancer with MDCT using the water-filling method and multiplanar reconstruction: CT-histologic correlation. AJR Am J Roentgenol. 2005;185:1152–1158. doi: 10.2214/AJR.04.0651. [DOI] [PubMed] [Google Scholar]
- 27.Blank S, Bläker H, Schaible A, Lordick F, Grenacher L, Buechler M, Ott K. Impact of pretherapeutic routine clinical staging for the individualization of treatment in gastric cancer patients. Langenbecks Arch Surg. 2012;397:45–55. doi: 10.1007/s00423-011-0805-8. [DOI] [PubMed] [Google Scholar]
- 28.Zheng L, Zou K, Yang C, Chen F, Guo T, Xiong B. Inflammation-based indexes and clinicopathologic features are strong predictive values of preoperative circulating tumor cell detection in gastric cancer patients. Clin Transl Oncol. 2017;19:1125–1132. doi: 10.1007/s12094-017-1649-7. [DOI] [PubMed] [Google Scholar]
- 29.Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Wang Y, Zhang X, Zhang T. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: Review and meta-analysis. Clin Chim Acta. 2018;486:303–310. doi: 10.1016/j.cca.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688–2692. doi: 10.1007/s00268-013-2156-9. [DOI] [PubMed] [Google Scholar]
- 32.Eo WK, Chang HJ, Suh J, Ahn J, Shin J, Hur JY, Kim GY, Lee S, Park S, Lee S. The Prognostic Nutritional Index Predicts Survival and Identifies Aggressiveness of Gastric Cancer. Nutr Cancer. 2015;67:1260–1267. doi: 10.1080/01635581.2015.1082112. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Kim HS, Seo WY, Nam CM, Kim KY, Jeung HC, Lai JF, Chung HC, Noh SH, Rha SY. External validation of nomogram for the prediction of recurrence after curative resection in early gastric cancer. Ann Oncol. 2012;23:361–367. doi: 10.1093/annonc/mdr118. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida H, Shimazu T, Kiyuna T, Marugame A, Yamashita Y, Cosatto E, Taniguchi H, Sekine S, Ochiai A. Automated histological classification of whole-slide images of gastric biopsy specimens. Gastric Cancer. 2018;21:249–257. doi: 10.1007/s10120-017-0731-8. [DOI] [PubMed] [Google Scholar]