Figure 3. MUC1-C forms a direct complex with MYC to activate MTA1, MBD3 and CHD4 expression.
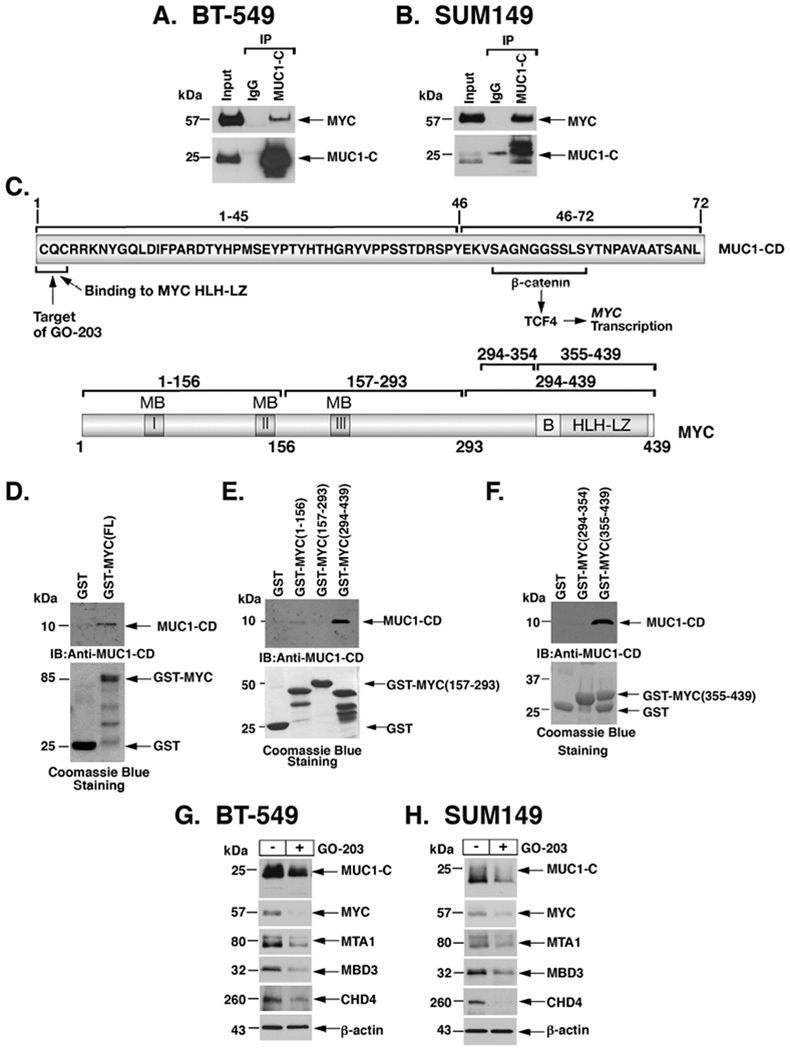
A-B. Nuclear lysates from BT-549 (A) and SUM149 (B) cells were incubated with anti-MUC1-C or a control IgG. The input and precipitates were analyzed by immunoblotting with anti-MYC and anti-MUC1-C. C. Upper panel: Schema of MUC1-C (ED, extracellular domain; TM, transmembrane domain) with the sequence of the 72-aa cytoplasmic domain (CD). Highlighted is the MUC1-C SAGNGGSSLS motif that binds directly to β-catenin and in a complex with TCF4 activates the MYC gene. Also highlighted is the MUC1-C CQC motif that is necessary for MUC1-C homodimerization and confers direct binding to the MYC helix-loop-helix-leucine zipper (HLH-LZ) domain. The MUC1-C CQC motif is the target of the GO-203 inhibitor, which blocks that site and thereby interactions with binding partners. Lower panel: Schema of the MYC protein. The MYC transactivation function is regulated by an unstructured N-terminal domain that contains MYC boxes (MBs) and a nuclear localization signal. The MYC C-terminal region includes a basic (B) region and a helix-loop-helix-leucine zipper (HLH-LZ) domain, which confers binding to HLH-containing partner proteins. D. GST and GST-MYC (full length; 1-439) were incubated with purified MUC1-CD. The adsorbates were immunoblotted with anti-MUC1-CD. Input of the GST proteins was assessed by Coomassie Blue staining. E and F. GST, GST-MYC (1-156), GST-MYC(157-293) and GST-MYC(294-439) were incubated with purified MUC1-CD (E). GST, GST-MYC(294-354) and GST-MYC(355-439) were incubated with purified MUC1-CD (F). The adsorbates were immunoblotted with anti-MUC1-CD. Input of the GST proteins was assessed by Coomassie Blue staining. G-H. BT-549 (G) and SUM149 (H) cells were left untreated or treated with 5 μM GO-203 for 48 h. Lysates were immunoblotted with antibodies against the indicated proteins.
