Abstract
Evidence has been accumulating for an immune-based component to the etiology of psychotic disorders. Advancements in diffusion magnetic resonance imaging (MRI) have enabled estimation of extracellular free water (FW), a putative biomarker of neuroinflammation. Furthermore, inflammatory processes may be associated with altered brain levels of metabolites, such as glutathione (GSH). Consequently, we sought to test the hypotheses that FW is increased and associated with decreased GSH in patients with first episode schizophrenia (SZ) compared to healthy controls (HC). SZ (n=36) and HC (n=40) subjects underwent a multi-shell diffusion MRI scan on a Siemens 3T scanner. 1H-MR spectroscopy data were acquired using a GSH-optimized MEGA-PRESS editing sequence and GSH/creatine ratios were calculated for DLPFC (SZ: n=33, HC: n=37) and visual cortex (SZ: n=29, HC: n=35) voxels. Symptoms and functioning were measured using the SANS, SAPS, BPRS and GSF/GRF. SZ demonstrated significantly elevated FW in whole-brain gray (p=.001) but not white matter (p=.060). There was no significant difference between groups in GSH in either voxel. However, there was a significant negative correlation between DLPFC GSH and both whole-brain and DLPFC-specific gray matter FW in SZ (r=−.48 and −.47, respectively; both p<.05), while this relationship was nonsignificant in HC and in both groups in visual cortex. These data illustrate an important relationship between a metabolite known to be important for immune function – GSH – and the diffusion extracellular FW measure, which provides additional support for these measures as neuroinflammatory biomarkers that could potentially provide tractable treatment targets to guide pharmacological intervention.
Introduction
Evidence has been accumulating for an immune-based component of schizophrenia (SZ) etiology. Mednick and colleagues 1 provided some of the first evidence many decades ago, identifying increased rates of psychotic disorders in the offspring of women who were exposed to infection while pregnant 2 see for review. In more recent years genetic links have been identified with the major histocompatibility complex, a section of the genome critical for immune function3. Furthermore, alterations in molecular markers mediating immune function, such as the cytokines IL-6, IFN-g, and TNF-a, have been repeatedly observed both in first episode and chronic individuals with schizophrenia, both on and off medication 4.
Recently, several groups have attempted to identify this “neuroinflammatory” signal using various brain imaging techniques. Initial studies using positron emission tomography and translocator protein (TSPO) ligands provided some evidence of increased microglial activity in gray matter and hippocampus 5, 6. However, more recent studies using second generation TSPO ligands, including some with larger samples, have not replicated this result 7–10.
Other putative markers of neuroinflammation may be measured using magnetic resonance imaging techniques, including diffusion imaging and proton spectroscopy. Pasternak and colleagues have recently demonstrated the utility of measuring extracellular free water, both as a method of adjusting for free water in the calculation of fractional anisotropy 11, but also as a separate biomarker that may be associated with inflammation or other neuropathological processes. This research group has demonstrated a pattern of increased free water and comparable white matter integrity in first episode psychosis samples 12, while a chronic sample showed more prominent reductions in fractional anisotropy 13. There is also preliminary evidence that chronically ill individuals with present state delusions show elevated free water compared to controls, particularly in the cingulum bundle, although free water did not distinguish those with present delusions from other patient groups with either past delusions or no lifetime history 14.
Furthermore, there is evidence that neuroimmune activation may alter brain levels of metabolites that can be measured non-invasively with proton magnetic resonance spectroscopy (1H-MRS). Glutathione (GSH) is a potentially informative inflammatory biomarker, as it is one of the major antioxidants in the human body and plays a role in cell proliferation, apoptosis, and immune function. GSH can be depleted by oxidative stress and is reduced in several neuroinflammatory conditions (e.g., multiple sclerosis, AIDS) 15, 16. In addition, recent mouse and in vitro studies have shown that GSH (directly and via its precursor, NAC) also has specific, protective interactions with neuroimmune response cascades, including inhibition of microglial activation and reduction of brain inflammatory markers evoked by neuroimmune activating agents 17, 18. One influential model that is supported by epidemiological studies as well as a large body of work using animal model systems posits that in some individuals, maternal immune activation, which may be related to maternal infections, exposures or other stressors in utero, causes a long-lasting sensitization of neuroimmune response elements in individuals who later develop schizophrenia. These sensitized systems might include the neuroimmune and proinflammatory functions of microglia and astrocytes. While GSH may not prevent this initial sensitization, it could plausibly protect against some of the consequences of such sensitization. Sensitization renders the neuroimmune response elements vulnerable to activation by normally subthreshold provocations. GSH could inhibit such activation, as has been shown with its precursor, NAC, for microglial activation by LPS 17. Consequently, GSH is a strong candidate biomarker for psychotic disorders and the majority of studies have found GSH to be reduced in blood 19–35 and to some degree in CSF 29, 36 compared to control subjects. Useful measurement of GSH in brain with 1H-MRS requires either high field MR systems not generally available in clinical centers or advanced acquisition sequences, such as J-difference editing 37–39. In the earliest study of GSH in prefrontal cortex, Do and colleagues 36 used a double quantum coherence sequence at 1.5 Tesla and reported reduced medial prefrontal cortex (mPFC) GSH in 14 schizophrenia patients compared to 14 comparison subjects. However, more recent studies using a STEAM sequence at 4 Tesla 40 and a MEGA-PRESS editing sequence at 3 Tesla 41 observed no group differences in mPFC GSH. A study by Wood and colleagues 42 reported increased medial temporal cortex GSH in a psychotic disorder sample. However, the use of a conventional PRESS sequence at 3 Tesla makes it challenging to interpret these data 39. Of the four largest and most recent studies, two showed significantly lower GSH in patients compared to controls using a STEAM sequence at 7 Tesla 43, 44, while the other two studies showed no differences between patients and controls 19, 45 . It is not clear why GSH reductions have not been consistently observed. Small sample sizes, varying clinical features of the patient groups, and different technical methods may account for some of these differences.
The goal of the present study is to leverage the use of these two biomarkers, extracellular free water and cortical GSH, to further investigate the role of immune disruption in the brain in individuals with a first episode psychotic disorder. Given that free water may be influenced by a variety of factors, including inflammation, degeneration, or edema, testing the relationship to an established measure of immune function in brain—GSH—will inform the validity and clinical utility of this measure. Building upon previously published single-shell diffusion studies in first episode psychosis 12 we used a multi-shell diffusion weighted acquisition optimized for the measurement of free water in brain tissue. We hypothesize that free water will be increased in individuals with first episode psychosis. We also predict that dorsolateral prefrontal cortex (DLPFC) GSH level will correlate negatively with free water in patients, reflecting a convergence of factors associated with neuroinflammation that varies in degree among individuals with first episode psychosis. In addition, we will test the hypothesis that GSH is reduced in the patient group as a whole.
Methods and Participants
Thirty-nine first episode schizophrenia patients (See Table 1 for diagnoses and clinical status) and 41 control subjects between the ages of 16 and 30 were recruited for the study. After exclusions for data quality, 36 schizophrenia and 40 control participants remained with good diffusion data. Of this sample, 33 patients and 37 controls had good quality DLPFC GSH data and 29 patients and 35 controls had good visual cortex GSH data (see Supplementary Information for details). Participants with schizophrenia-spectrum diagnoses were outpatients within two years of their first psychotic episode. All participants were assessed using the Structured Clinical Interview for the DSM-IV-TR SCID-I/P; 46. Clinical ratings were collected in the patient group using the Scale for the Assessment of Negative Symptoms SANS; 47, Scale for the Assessment of Positive Symptoms SAPS; 48, Brief Psychiatric Rating Scale BPRS; 49, and Global Functioning: Social 50 and Role 51 scales. Exclusion criteria for both groups included: Wechsler Abbreviated Scale of Intelligence (WASI) IQ score below 70, alcohol or drug dependence or abuse within 3 months before testing, positive urine toxicology screen for illicit drugs, prior head trauma worse than a Grade I concussion, or contraindication to MRI scanning. Control subjects were excluded for the following additional criteria: any lifetime diagnosis of an Axis I or Axis II disorder or any first-degree relatives with a psychotic disorder. Before testing, a detailed description of the study was provided and written informed consent obtained. The study was approved by the University of California, Davis Institutional Review Board and all subjects consented to and were paid for their participation.
Table 1:
Sample demographic and clinical characteristics.
| Control (n=40) | Schizophrenia (n=36) | |
|---|---|---|
| Age: mean (SD) | 21.9 (2.8) | 21.4 (3.4) |
| Gender: number Male/Female | 27 / 13 | 25 / 11 |
| Education: mean (SD) | 14.3 (2.6) | 12.5 (1.9)* |
| Parental Education: mean (SD) | 14.7 (3.5) | 14.4 (2.9) |
| WASI: mean (SD) | 115.8 (12.3) | 104.6 (18.6)* |
| Diagnosis: (n) | ||
| Schizophreniform | - | 3 |
| Schizophrenia | - | 18 |
| Schizoaffective | - | 15 |
| Antipsychotic Dose: mean CPZ equivalent (SD) | - | 226.64 (168.7) |
| SANS | - | 11.2 (3.6) |
| SAPS | - | 4.4 (3.4) |
| BPRS | - | 46.8 (12.0) |
| Global Functioning: Social | - | 6.1 (1.5) |
| Global Functioning: Role | - | 3.5 (2.5) |
SD: Standard Deviation; WASI: Wechsler Abbreviated Scale of Intelligence; CPZ: chlorpromazine; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms; BPRS: Brief Psychiatric Rating Scale.
p<.05.
Imaging Parameters and Data Analysis
Imaging data were obtained using a 3T Siemens Tim Trio MRI scanner. T1-weighted MPRAGE structural images were acquired with the following settings: TR=2,530-msec, echo time=3.5-msec, flip-angle=7˚, field of view=256mm, 1mm isotropic voxels. 1H-MRS data were acquired from voxels placed in the DLPFC and in the visual cortex, as a control region (see Supplementary Information). GSH was measured with a MEGA-PRESS, J-difference, editing sequence optimally adapted for GSH 37, 52–54. Parameters included: TE/TR = 131/2000; bandwidth = 2000 Hz; on and off resonance SLR edit pulse frequencies = 4.56 and 4.90 ppm; edit pulse bandwidth = 30 Hz; edit pulse duration = 39.68 msec. The edited spectra were acquired in two sequential acquisitions of 176 averages each (total 352 averages, 11.7 minutes total duration). A conventional PRESS sequence was acquired from the same voxels with TE/TR = 30/1500 and 160 averages.
The diffusion sequence was acquired with the following settings: TR=11,400-msec, echo time=92.4-msec, field of view=240mm, 1.7mm isotropic voxels. The sequence included 56 directions acquired P-A with the following b-shells: 12 x b=0, 10 x b=500, 30 x b=900, 16 x b=1400. Diffusion images were first visually inspected for image quality and images containing artifacts were discarded. The remaining diffusion weighted images underwent eddy current correction and realignment using FSL’s eddy 55, including rotation of b-matrices. The Dipy diffusion imaging library 56 and included free water elimination model 57 were used to calculate all diffusion metrics. This model expands the typical DTI model and assumes that each voxel contains two components: an anisotropic tissue-bound component and an isotropic extracellular free water component. In this study, we evaluated both the free water component and tissue-specific fractional anisotropy (FA-t), which reflects traditional FA with the free water component eliminated.
In order to calculate free water values specific to gray and white matter, brain extraction and segmentation of MPRAGE images was performed using Freesurfer 5.3 58. White and gray matter masks from Freesurfer were brought into alignment with each subject’s free water image using bbregister 59. Each MPRAGE underwent a rigorous quality inspection by individuals blind to group—including talairach alignment evaluation, meninges/skull removal, white matter editing, and surface inspection, according to standard Freesurfer documentation. Mean free water values were calculated across all voxels within gray and white matter masks for each subject.
To further explore the regional specificity of gray and white matter free water, two additional analyses were performed (see Supplement). Briefly, free water maps were projected onto individual subject cortical surfaces using Freesurfer’s mri_vol2surf to assess free water in a vertex-wise manner using mri_glmfit. Multiple comparisons correction was employed using cluster analysis with Freesurfer’s precomputed Z Monte Carlo simulation (cluster threshold of p<0.01 and clusterwise probability of p<0.05). White-matter free water and FA-t maps were nonlinearly aligned to the FMRIB58_FA image using FNIRT and projected on the white matter skeleton using FSL’s Tract Based Spatial Statistics (TBSS) 60. Randomise61 and threshold-free cluster enhancement62 were used to define clusters and correct for multiple comparisons.
GSH was quantified by peak integration of the MEGA-PRESS difference spectra using LCModel 6.3–1L 63, jMRUI 5.2 64 and custom software in an operator-independent sequence of processing steps. The on and off resonance spectra from each acquisition were first zero-filled (2x), phase-aligned, and apodized in jMRUI. The on and off resonance spectra were then frequency-aligned using custom software and subtracted to generate difference spectra. The two difference spectra were phase and frequency aligned to each other and summed. The GSH cysteine resonance at 2.95 ppm was then quantified by line-width optimized peak integration in the final difference spectrum. The creatine resonance at 3.02 ppm was similarly quantified by peak integration in the final summed spectrum and used to calculate the GSH/creatine ratio (Supplementary Figure 1). To explore the specificity of the correlation between free water and GSH, five supplementary metabolites (total choline, total NAA, total creatine, glutamate, and inositol) from the PRESS acquisition were tested for relationships with whole-brain gray/white free water values (see Supplement).
Statistical Methods
Differences in demographic characteristics were assessed with Pearson Chi-Square or independent samples t-tests, where appropriate. Between-group free water and GSH/creatine ratios were assessed with independent samples t-tests and relationships between free water and GSH/creatine ratios were tested with Pearson r correlations. Any measures that were not normally distributed underwent follow-up non-parametric tests and are reported if significance changed. Alpha was set at p<.05, two-tailed, to determine significance for all tests.
Results
Participant demographic and clinical information is presented in Table 1. The groups did not differ significantly on age (t(74)=.70, p=.48), gender (χ2=.033, p=.86), or parental education (t(71)=.42, p=.67). Controls were more highly educated than patients (t(74)=3.37, p=.001) and showed significantly higher estimated IQ (t(71)=3.05, p=.003).
Between-group Comparisons
As seen in Figure 1, individuals with first episode schizophrenia demonstrated significantly elevated free water in whole-brain gray (t(74)=3.79, p<.001) with a trend elevation in white matter (t(74)=1.91, p=.060). There were no significant group differences in GSH within the DLPFC (t(68)= 1.07, p=.29) or visual cortex voxels (t(62)=.19, p=.85). There was a significant negative correlation (Figure 2) between DLPFC GSH and gray matter free water in the patient group (r=−.48, p=.004), although the relationship with white matter free water did not reach significance (r=−.26, p=.14). Free water was not correlated to GSH in controls or any visual cortex data. Finally, the relationship between DLPFC GSH and gray matter free water was significantly stronger in first episode participants compared to controls (Fisher’s r-to-z, p=.005). The strength of the free water-GSH relationship did not differ between groups in the other three comparisons (all p>.32).
Figure 1:
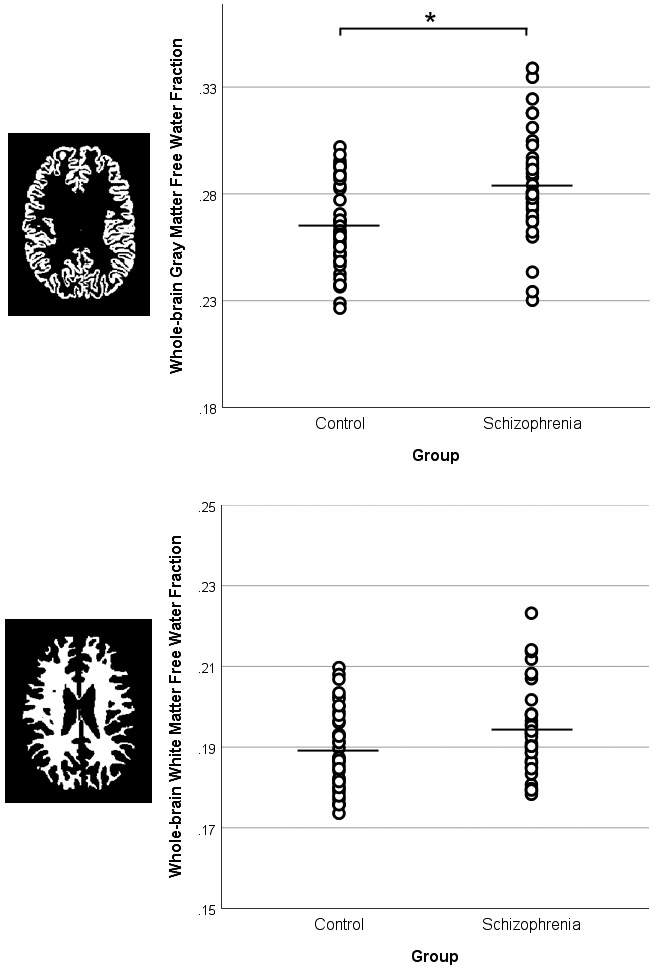
Whole-brain gray and white matter free water values. Independent samples t-test revealed increased free water in first episode schizophrenia patients (SZ) compared to controls (HC) in gray matter (p<.001), with a trend elevation in white matter (p=.060). Black lines represent the mean. Means and standard deviations are presented in Supplementary Table 3.
Figure 2:
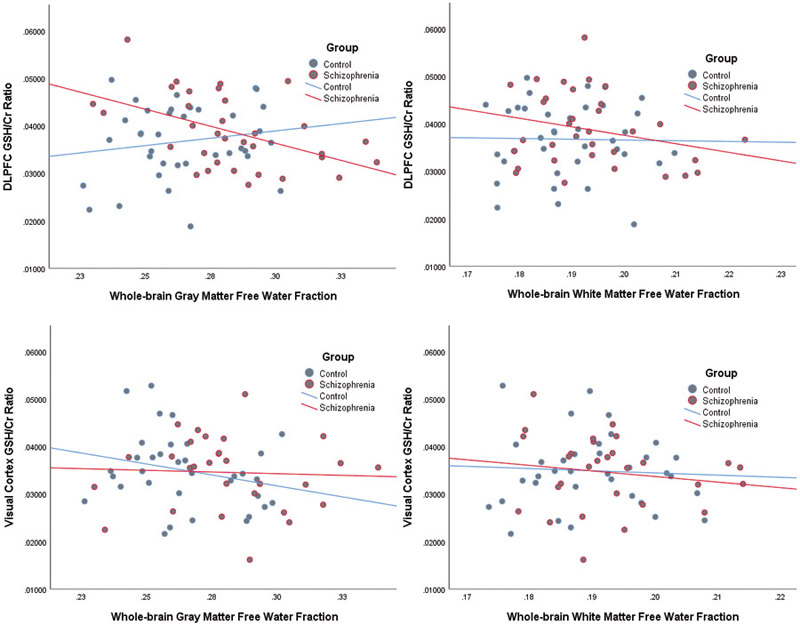
Relationships between glutathione (GSH) and free water measures were tested with Pearson r correlations. The top two panels present the relationship between gray (top left) and white (top right) free water and DLPFC GSH values. The bottom two panels present the relationship between gray (bottom left) and white (bottom right) free water and visual cortex GSH values. A significant inverse relationship was identified between GSH in the hypothesized DLPFC region and gray matter free water in schizophrenia patients (p=0.004). Additionally, the relationship between GSH and gray matter free water was stronger in the schizophrenia group compared to the control group (Fisher’s r-to-z, p=.005).
A follow-up analysis was also performed on free water in tissue specifically restricted to the MRS voxel location and detailed in the Supplement. In brief, these results are very similar to the whole-brain findings, with a significant relationship in patients between DLPFC voxel-specific gray matter free water and DLPFC GSH (r=−.472, p=.006), with a significantly stronger relationship in patients compared to controls (Fisher’s r-to-z, p=.02).
Voxel-wise TBSS analyses revealed significantly increased free water in patients with schizophrenia compared to controls (Figure 3) across both hemispheres in a relatively widespread pattern. No group differences were identified in the voxel-wise FA-t TBSS analysis.
Figure 3:
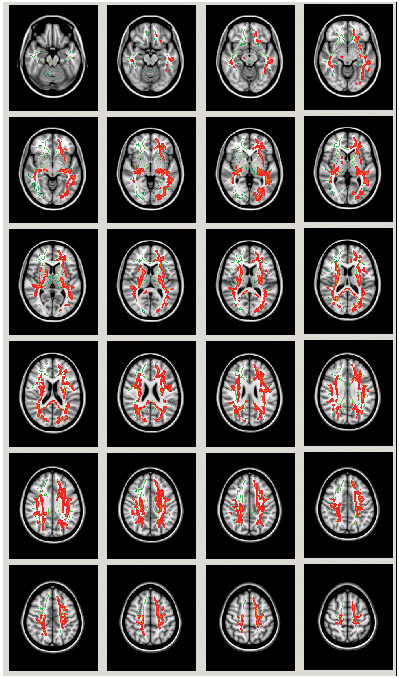
Tract-based spatial statistics voxel-wise analysis of free water group differences presented in axial slices. Regions in red depict areas in which first episode schizophrenia patients showed significantly higher free water compared to controls. Clusters presented survive correction for multiple comparisons (p<.05).
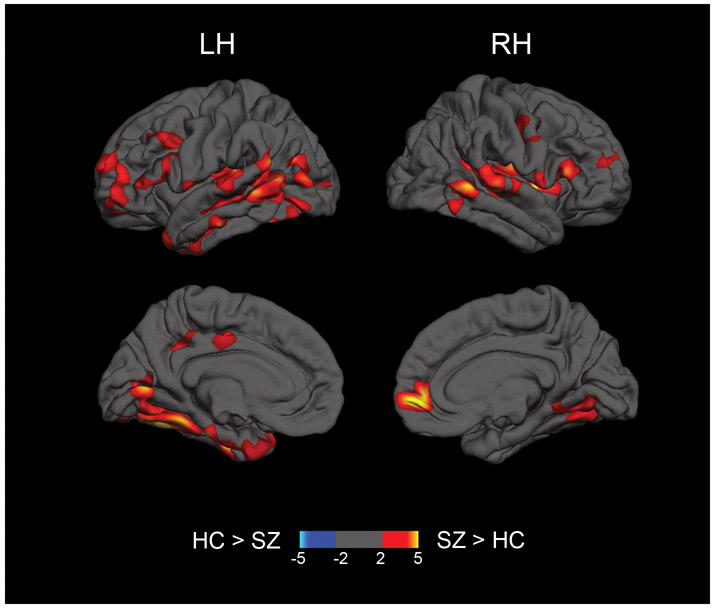
Vertex-wise analyses of gray matter free water projected to the cortical surface revealed significantly increased free water in patients with schizophrenia in lateral frontal cortex, right rostral anterior cingulate, bilateral temporal cortex extending into the insula, left hemisphere inferior parietal cortex, left hemisphere posterior cingulate, and occipital cortex (Figure 4 and Supplementary Table 1).
Figure 4:
Representation of regional group differences in free water in the cortical gray matter. Free water was projected on the cortical surface and tested for group differences using Freesurfer tools. Hot colors represent clusters in which first episode schizophrenia patients (SZ) show significantly higher free water compared to controls (HC) in the left (LH) and right (RH) hemispheres. Clusters presented survive correction for multiple comparisons (p<.05). The color scale ranges from 2 to 5, representing −log10(p-value) of p=.01 to .00001.
Specificity of GSH-Free Water Relationship
Potential concerns in testing only the relationship between GSH and free water are that the association could potentially be driven by creatine-associated variance in the GSH/Cr ratio or that the GSH-free water association is not specific to GSH. For example, inositol has also been linked to neuroimmune alterations 65–67. To explore these issues, we evaluated the relationship between free water and the other reliably estimated creatine normalized metabolites (total choline, NAA, glutamate, inositol, and the absolute value of creatine; all CRLB < 10%) from the DLPFC PRESS acquisition. None of these metabolites significantly correlated with gray or white matter free water in either group (all p > .13), suggesting a specific relationship with GSH.
Relationships to Symptoms and Functioning
Clinical scores were included in vertex- and voxel-wise whole-brain regressions with free water using Freesurfer and TBSS. Significant positive associations were identified between prefrontal gray matter free water (rostral middle frontal gyrus and anterior cingulate) and both SAPS and BPRS total scores (Supplementary Figure 3 and Supplementary Table 2). No voxels survived correction for multiple comparisons in white matter free water-symptom analyses.
Discussion
These data represent the first evaluation of both GSH and multi-shell diffusion extracellular free water in a first episode schizophrenia sample. In agreement with results reported by Pasternak et al. 12, free water was elevated in individuals with first episode psychosis in both white and gray matter. Furthermore, while the groups did not differ on GSH, the significant inverse relationship observed between GSH and gray matter free water could reflect a common linkage to neuroinflammatory processes. Consistent with most prior studies, prefrontal GSH levels were not different between psychosis and control groups. While free water could represent an amalgam of biological processes, including inflammation, degeneration, or edema, the significant relationship to a known inflammation-related metabolite, GSH, provides additional support for the link between free water and neuroimmune processes and the use of the combination of measures as complementary biomarkers to guide pharmacological intervention.
Several studies using single-shell diffusion data have shown that extracellular brain free water is increased in patients with first episode schizophrenia 12, 68 and, to a lesser degree, chronic schizophrenia 13, 69. We show similar increases in free water throughout the white matter skeleton using an optimized multi-shell sequence, which should represent an improvement in estimating free water. Furthermore, we used the novel approach of evaluating gray matter free water projected on the cortical surface, which identified lateral and medial prefrontal, temporal/insula, inferior parietal, and occipital clusters of increased free water in the schizophrenia sample. These specific regions of elevated free water notably overlap with regional disruptions of gray matter density 70, 71, cortical thickness 72, 73, and fMRI BOLD 74 activity that are repeatedly identified in individuals with schizophrenia. Notably, we also found that higher free water in prefrontal gray matter was also associated with worse symptomatology, suggesting that this measure may index clinical severity.
Although free water was the primary diffusion metric for the present study, we also investigated FA-t in order to evaluate microstructural white matter integrity between groups. Our analyses revealed no significant differences in FA-t throughout the white matter skeleton. While the majority of published studies suggest decreases in fractional anisotropy 75–77 only more recent studies have applied the free water elimination model to evaluate FA-t. This is critically important, given that free water contamination of voxels may artificially lower traditional FA values in individuals who have more free water. Specifically, the studies that evaluated FA-t in first episode samples identified relatively isolated FA-t decreases 12, 68 in contrast to the more widespread traditional FA decreases in chronic schizophrenia. The young, first episode sample in the present study, with a short duration of illness, treated with relatively low doses of antipsychotics, and an absence of substance abuse may also partially explain the lack of FA-t differences.
Our finding of similar levels of GSH in patient and control groups is relatively consistent with the GSH literature, given that the majority of published studies have also shown no significant differences 19, 40, 41, 45. Since the pioneering work of Do and colleagues 36, there have been only two additional published reports of lowered brain GSH in schizophrenia 43, 44, although a negative relationship between GSH levels and negative symptoms has been reported 41.
Interestingly, the relationship between GSH and free water was only identified in individuals with first episode psychosis and only in the DLPFC voxel. In addition, the specific relationship between DLPFC GSH and gray matter free water was significantly stronger in the patient group compared to controls. One interpretation of these findings is that this relationship may emerge only in the presence of an inflammatory process. Additionally, the lack of any relationships between free water and the visual cortex control region suggests that there may be some regional specificity to these findings, and add to the large body of existing literature demonstrating DLPFC dysfunction across many imaging modalities in first episode schizophrenia. Several interacting homeostatic mechanisms regulate brain tissue GSH levels, tending to keep them within a range of normal values 78, 79. Some inflammatory processes can alter this process sufficiently to cause low GSH levels, such as in multiple sclerosis 80, 81. Some inflammatory processes do not have this effect, and GSH levels remain normal, as in ALS, for example 82, 83. It is possible that an inflammation-related process in schizophrenia could cause elevated free water without causing reduced GSH at the mean group level, as is suggested by our data. In this case, GSH could be serving a protective function. Those patients who maintain a relatively high GSH level may benefit from greater protection against inflammation compared to those patients who maintain a relatively low GSH level. In the absence of an underlying inflammatory process in the control group, GSH levels are not expected to correlate inversely with free water values in this group. This is because GSH is not serving a protective role against a pathogenic process causing elevated free water in this group.
Limitations
GSH is a difficult metabolite to measure, particularly at lower field strengths. Our approach was to use an optimized MEGA-PRESS sequence at 3 Tesla and to quantify GSH relative to creatine. It is possible that more sensitive measures of GSH or larger sample sizes might have revealed further associations with GSH levels. There have been reports in the literature that creatine levels may be reduced in schizophrenia 84, although a recent meta-analysis found no evidence for decreased creatine in schizophrenia or bipolar disorder 85. Lower creatine levels in the patient group would tend to skew results in the opposite direction of our hypotheses, with the patient group potentially showing a bias towards a higher GSH/creatine ratio. Given that our findings reveal no significant differences between groups, it remains possible, although unlikely, that differences in creatine may have contributed to the null result. However, an exploratory evaluation of other metabolites normalized to creatine revealed a specific relationship with the GSH/creatine ratio and free water, which suggests creatine is not the main driver of these findings. An additional concern is that neuroinflammation is only one possible mechanism that could account for the pattern of findings we report here. For instance, increases in free water could reflect other neuropathological processes unrelated to immune processes, although the lack of group differences in FA-t suggests that free water increases are not likely due to white matter degradation. An additional concern is that the majority of subjects in the sample were taking antipsychotic medication and a growing body of literature suggests that antipsychotics may have anti-inflammatory properties. Recent meta-analyses of the cytokine literature 4, 86 highlight antipsychotic-related decreases of pro-inflammatory cytokines in the peripheral circulation, particularly IL-1B, IL-6, and IFN-g. Additionally, antipsychotics have been associated with inhibition of these same pro-inflammatory cytokines in stimulated microglia cultures 87. Additional work, particularly in animal models and antipsychotic-naïve individuals, is needed to investigate these relationships.
Conclusions and Future Directions
These data provide compelling evidence that the simultaneous acquisition of different imaging measures including extracellular free water and GSH can inform our understanding of altered neuroinflammatory processes that may underlie the developmental biology of psychotic disorders. However, the overlap between control and patient groups in both free water and GSH values is considerable and consequently only a subset of patients may evidence a strong immune component to the etiology of their illness. Indeed, post mortem analyses in schizophrenia have suggested that there is increased expression of inflammation-related genes in perhaps thirty 88 to forty 89 percent of patients. This heterogeneity may partly explain why several clinical trials evaluating add-on treatment using anti-inflammatory agents have shown relatively modest benefits in relieving symptomatology 90–95, with one additional study showing no benefit 96. Consequently, the use of MRI-based markers such as those used in the present study may offer the potential of a more personalized approach to clinical trials of inflammation-related therapies, in which patients are stratified based upon diffusion MRI data and offered targeted intervention based upon these measures.
Supplementary Material
Acknowledgements
This study was funded by National Institutes of Health P50MH106438 and R01MH059883 grants awarded to C.S.C. The authors would like to thank Dr. Thorsten Feiweier from Siemens AG, Healthcare for providing the prototype software package for advanced diffusion imaging which was used to acquire data in the present study and Michael Maddock for assistance in development of custom software for MRS data processing.
Footnotes
Conflict of Interest
The authors declare no conflicts of interests.
Supplementary information is available at MP’s website.
References
- 1.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry 1988; 45(2): 189–192. [DOI] [PubMed] [Google Scholar]
- 2.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167(3): 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016; 530(7589): 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2011; 70(7): 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 2009; 50(11): 1801–1807. [DOI] [PubMed] [Google Scholar]
- 6.van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 2008; 64(9): 820–822. [DOI] [PubMed] [Google Scholar]
- 7.Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 2010; 13(7): 943–950. [DOI] [PubMed] [Google Scholar]
- 8.Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP et al. Imaging Microglial Activation in Untreated First-Episode Psychosis: A PET Study With [(18)F]FEPPA. Am J Psychiatry 2017; 174(2): 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collste K, Plaven-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A et al. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [(11)C]PBR28. Mol Psychiatry 2017; 22(6): 850–856. [DOI] [PubMed] [Google Scholar]
- 10.van der Doef TF, de Witte LD, Sutterland AL, Jobse E, Yaqub M, Boellaard R et al. In vivo (R)-[(11)C]PK11195 PET imaging of 18kDa translocator protein in recent onset psychosis. NPJ Schizophr 2016; 2: 16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med 2009; 62(3): 717–730. [DOI] [PubMed] [Google Scholar]
- 12.Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci 2012; 32(48): 17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasternak O, Westin CF, Dahlben B, Bouix S, Kubicki M. The extent of diffusion MRI markers of neuroinflammation and white matter deterioration in chronic schizophrenia. Schizophr Res 2015; 161(1): 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oestreich LK, Pasternak O, Shenton ME, Kubicki M, Gong X, Australian Schizophrenia Research B et al. Abnormal white matter microstructure and increased extracellular free-water in the cingulum bundle associated with delusions in chronic schizophrenia. Neuroimage Clin 2016; 12: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buhl R, Jaffe HA, Holroyd KJ, Wells FB, Mastrangeli A, Saltini C et al. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet 1989; 2(8675): 1294–1298. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho AN, Lim JL, Nijland PG, Witte ME, Van Horssen J. Glutathione in multiple sclerosis: more than just an antioxidant? Mult Scler 2014; 20(11): 1425–1431. [DOI] [PubMed] [Google Scholar]
- 17.Markoutsa E, Xu P. Redox Potential-Sensitive N-Acetyl Cysteine-Prodrug Nanoparticles Inhibit the Activation of Microglia and Improve Neuronal Survival. Mol Pharm 2017; 14(5): 1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Xu S, Huang Q, Xu H. N-acetylcysteine attenuates the cuprizone-induced behavioral changes and oligodendrocyte loss in male C57BL/7 mice via its anti-inflammation actions. J Neurosci Res 2018; 96(5): 803–816. [DOI] [PubMed] [Google Scholar]
- 19.Xin L, Mekle R, Fournier M, Baumann PS, Ferrari C, Alameda L et al. Genetic Polymorphism Associated Prefrontal Glutathione and Its Coupling With Brain Glutamate and Peripheral Redox Status in Early Psychosis. Schizophr Bull 2016; 42(5): 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballesteros A, Jiang P, Summerfelt A, Du X, Chiappelli J, O’Donnell P et al. No evidence of exogenous origin for the abnormal glutathione redox state in schizophrenia. Schizophr Res 2013; 146(1–3): 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Liencres C, Tas C, Brown EC, Erdin S, Onur E, Cubukcoglu Z et al. Oxidative stress in schizophrenia: a case-control study on the effects on social cognition and neurocognition. BMC Psychiatry 2014; 14: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mico JA, Rojas-Corrales MO, Gibert-Rahola J, Parellada M, Moreno D, Fraguas D et al. Reduced antioxidant defense in early onset first-episode psychosis: a case-control study. BMC Psychiatry 2011; 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langbein K, Hesse J, Gussew A, Milleit B, Lavoie S, Amminger GP et al. Disturbed glutathione antioxidative defense is associated with structural brain changes in neuroleptic-naive first-episode psychosis patients. Prostaglandins Leukot Essent Fatty Acids 2018; 136: 103–110. [DOI] [PubMed] [Google Scholar]
- 24.Altuntas I, Aksoy H, Coskun I, Caykoylu A, Akcay F. Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients. Clin Chem Lab Med 2000; 38(12): 1277–1281. [DOI] [PubMed] [Google Scholar]
- 25.Raffa M, Atig F, Mhalla A, Kerkeni A, Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry 2011; 11: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietrich-Muszalska A, Olas B, Glowacki R, Bald E. Oxidative/nitrative modifications of plasma proteins and thiols from patients with schizophrenia. Neuropsychobiology 2009; 59(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 27.Nucifora LG, Tanaka T, Hayes LN, Kim M, Lee BJ, Matsuda T et al. Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Transl Psychiatry 2017; 7(8): e1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33(7): 1178–1183. [DOI] [PubMed] [Google Scholar]
- 29.Samuelsson M, Skogh E, Lundberg K, Vrethem M, Ollinger K. Taurine and glutathione in plasma and cerebrospinal fluid in olanzapine treated patients with schizophrenia. Psychiatry Res 2013; 210(3): 819–824. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Litago F, Seco J, Echevarria E, Martinez-Cengotitabengoa M, Gil J, Irazusta J et al. Adaptive response in the antioxidant defence system in the course and outcome in first-episode schizophrenia patients: a 12-months follow-up study. Psychiatry Res 2012; 200(2–3): 218–222. [DOI] [PubMed] [Google Scholar]
- 31.Raffa M, Barhoumi S, Atig F, Fendri C, Kerkeni A, Mechri A. Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. Prog Neuropsychopharmacol Biol Psychiatry 2012; 39(2): 371–375. [DOI] [PubMed] [Google Scholar]
- 32.Vidovic B, Stefanovic A, Milovanovic S, Ethordevic B, Kotur-Stevuljevic J, Ivanisevic J et al. Associations of oxidative stress status parameters with traditional cardiovascular disease risk factors in patients with schizophrenia. Scand J Clin Lab Invest 2014; 74(3): 184–191. [DOI] [PubMed] [Google Scholar]
- 33.Al-Asmari AK, Khan MW. Inflammation and schizophrenia: alterations in cytokine levels and perturbation in antioxidative defense systems. Hum Exp Toxicol 2014; 33(2): 115–122. [DOI] [PubMed] [Google Scholar]
- 34.Fukushima T, Iizuka H, Yokota A, Suzuki T, Ohno C, Kono Y et al. Quantitative analyses of schizophrenia-associated metabolites in serum: serum D-lactate levels are negatively correlated with gamma-glutamylcysteine in medicated schizophrenia patients. PLoS One 2014; 9(7): e101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai MC, Liou CW, Lin TK, Lin IM, Huang TL. Changes in oxidative stress markers in patients with schizophrenia: the effect of antipsychotic drugs. Psychiatry Res 2013; 209(3): 284–290. [DOI] [PubMed] [Google Scholar]
- 36.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci 2000; 12(10): 3721–3728. [DOI] [PubMed] [Google Scholar]
- 37.Terpstra M, Henry PG, Gruetter R. Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn Reson Med 2003; 50(1): 19–23. [DOI] [PubMed] [Google Scholar]
- 38.Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Oz G et al. N-Acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol 2013; 36(4): 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nezhad FS, Anton A, Parkes LM, Deakin B, Williams SR. Quantification of glutathione in the human brain by MR spectroscopy at 3 Tesla: Comparison of PRESS and MEGA-PRESS. Magnetic Resonance in Medicine 2016: 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terpstra M, Vaughan TJ, Ugurbil K, Lim KO, Schulz SC, Gruetter R. Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: application to schizophrenia. MAGMA 2005; 18(5): 276–282. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS One 2008; 3(4): e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood SJ, Berger GE, Wellard RM, Proffitt TM, McConchie M, Berk M et al. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiol Dis 2009; 33(3): 354–357. [DOI] [PubMed] [Google Scholar]
- 43.Kumar J, Liddle EB, Fernandes CC, Palaniyappan L, Hall EL, Robson SE et al. Glutathione and glutamate in schizophrenia: a 7T MRS study. Mol Psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang AM, Pradhan S, Coughlin JM, Trivedi A, DuBois SL, Crawford JL et al. Assessing Brain Metabolism With 7-T Proton Magnetic Resonance Spectroscopy in Patients With First-Episode Psychosis. JAMA Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandt AS, Unschuld PG, Pradhan S, Lim IA, Churchill G, Harris AD et al. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: A (1)H MRS Study at 7 Tesla. Schizophr Res 2016; 172(1–3): 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Biometrics Research, New York State Psychiatric Institute: New York, 2002. [Google Scholar]
- 47.Andreasen N The Scale for the Assessment of Negative Symptoms (SANS). . The University of Iowa: Iowa City, IA, 1983. [Google Scholar]
- 48.Andreasen N The Scale for the Assessment of Positive Symptoms (SAPS). . The University of Iowa: Iowa City, IA, 1984. [Google Scholar]
- 49.Lukoff D, Nuechterlein KH, Ventura J. Manual for the Expanded Brief Psychiatric Rating Scale (BPRS). Schizophr Bull 1986; 12: 594–602. [Google Scholar]
- 50.Auther AM, Smith CW, Cornblatt BA. Global Functioning: Social Scale (GF: Social). Zucker-Hillside Hospital: Glen Oaks, NY, 2006. [Google Scholar]
- 51.Niendam TA, Bearden CE, Johnson JK, Cannon TD. Global Functioning: Role Scale (GF: Role). University of California, Los Angeles: Los Angeles, 2006. [Google Scholar]
- 52.An L, Zhang Y, Thomasson DM, Latour LL, Baker EH, Shen J et al. Measurement of glutathione in normal volunteers and stroke patients at 3T using J-difference spectroscopy with minimized subtraction errors. J Magn Reson Imaging 2009; 30(2): 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 1998; 11(6): 266–272. [DOI] [PubMed] [Google Scholar]
- 54.Sanaei Nezhad F, Anton A, Parkes LM, Deakin B, Williams SR. Quantification of glutathione in the human brain by MR spectroscopy at 3 Tesla: Comparison of PRESS and MEGA-PRESS. Magn Reson Med 2017; 78(4): 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016; 125: 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform 2014; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoy AR, Koay CG, Kecskemeti SR, Alexander AL. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. Neuroimage 2014; 103: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9(2): 179–194. [DOI] [PubMed] [Google Scholar]
- 59.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009; 48(1): 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31(4): 1487–1505. [DOI] [PubMed] [Google Scholar]
- 61.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002; 15(1): 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44(1): 83–98. [DOI] [PubMed] [Google Scholar]
- 63.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001; 14(4): 260–264. [DOI] [PubMed] [Google Scholar]
- 64.Stefan D, Di Cesare F, Andrasescu A, Popa E, Lazariev A, Vescovo E et al. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas Sci Technol 2009; 20(10). [Google Scholar]
- 65.Schneider P, Weber-Fahr W, Schweinfurth N, Ho YJ, Sartorius A, Spanagel R et al. Central metabolite changes and activation of microglia after peripheral interleukin-2 challenge. Brain Behav Immun 2012; 26(2): 277–283. [DOI] [PubMed] [Google Scholar]
- 66.Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 2013; 8(3): 576–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Top Behav Neurosci 2012; 11: 199–251. [DOI] [PubMed] [Google Scholar]
- 68.Lyall AE, Pasternak O, Robinson DG, Newell D, Trampush JW, Gallego JA et al. Greater extracellular free-water in first-episode psychosis predicts better neurocognitive functioning. Mol Psychiatry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oestreich LKL, Lyall AE, Pasternak O, Kikinis Z, Newell DT, Savadjiev P et al. Characterizing white matter changes in chronic schizophrenia: A free-water imaging multi-site study. Schizophr Res 2017; 189: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bora E, Pantelis C. Structural trait markers of bipolar disorder: disruption of white matter integrity and localized gray matter abnormalities in anterior fronto-limbic regions. Biol Psychiatry 2011; 69(4): 299–300. [DOI] [PubMed] [Google Scholar]
- 71.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry 2008; 64(9): 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex 2005; 15(6): 708–719. [DOI] [PubMed] [Google Scholar]
- 73.Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ Jr., Pung CJ et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry 2010; 68(1): 41–50. [DOI] [PubMed] [Google Scholar]
- 74.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 2009; 66(8): 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karlsgodt KH. Diffusion Imaging of White Matter In Schizophrenia: Progress and Future Directions. Biol Psychiatry Cogn Neurosci Neuroimaging 2016; 1(3): 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 2009; 108(1–3): 3–10. [DOI] [PubMed] [Google Scholar]
- 78.Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci 2008; 108(3): 227–238. [DOI] [PubMed] [Google Scholar]
- 79.McBean GJ. Cysteine, Glutathione, and Thiol Redox Balance in Astrocytes. Antioxidants (Basel) 2017; 6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi IY, Lee SP, Denney DR, Lynch SG. Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3 T. Mult Scler 2011; 17(3): 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi IY, Lee P, Hughes AJ, Denney DR, Lynch SG. Longitudinal changes of cerebral glutathione (GSH) levels associated with the clinical course of disease progression in patients with secondary progressive multiple sclerosis. Mult Scler 2017; 23(7): 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheong I, Marjanska M, Deelchand DK, Eberly LE, Walk D, Oz G. Ultra-High Field Proton MR Spectroscopy in Early-Stage Amyotrophic Lateral Sclerosis. Neurochem Res 2017; 42(6): 1833–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Atassi N, Xu M, Triantafyllou C, Keil B, Lawson R, Cernasov P et al. Ultra high-field (7tesla) magnetic resonance spectroscopy in Amyotrophic Lateral Sclerosis. PLoS One 2017; 12(5): e0177680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ongur D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res 2009; 172(1): 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D et al. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res 2012; 203(2–3): 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tourjman V, Kouassi E, Koue ME, Rocchetti M, Fortin-Fournier S, Fusar-Poli P et al. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: A meta-analysis. Schizophr Res 2013; 151(1–3): 43–47. [DOI] [PubMed] [Google Scholar]
- 87.Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci 2009; 63(3): 257–265. [DOI] [PubMed] [Google Scholar]
- 88.Horvath S, Mirnics K. Immune system disturbances in schizophrenia. Biol Psychiatry 2014; 75(4): 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 2013; 18(2): 206–214. [DOI] [PubMed] [Google Scholar]
- 90.Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry 2002; 159(6): 1029–1034. [DOI] [PubMed] [Google Scholar]
- 91.Muller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M et al. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res 2010; 121(1–3): 118–124. [DOI] [PubMed] [Google Scholar]
- 92.Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res 2007; 90(1–3): 179–185. [DOI] [PubMed] [Google Scholar]
- 93.Chaudhry IB, Hallak J, Husain N, Minhas F, Stirling J, Richardson P et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol 2012; 26(9): 1185–1193. [DOI] [PubMed] [Google Scholar]
- 94.Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry 2010; 71(2): 138–149. [DOI] [PubMed] [Google Scholar]
- 95.Nitta M, Kishimoto T, Muller N, Weiser M, Davidson M, Kane JM et al. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull 2013; 39(6): 1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D. Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry 2005; 57(12): 1594–1596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.