Figure 2.
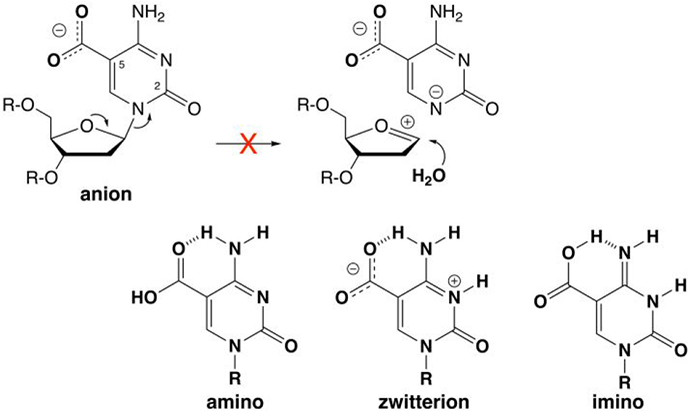
The caC base is a monoanion at physiological pH and N-glycosyl bond hydrolysis is likely precluded by the poor leaving-group (LG) quality of a departing caC dianion. For clarity, the focus is on LG departure in a stepwise mechanism (oxacarbenium ion intermediate), without showing details for nucleophile addition (see text). Leaving group quality is improved by protonation of the caC anion to give a neutral species (amine, zwitterion, or imino), as shown by previously calculated N1 acidities, where the free energy of deprotonation (kcal mol−1) in water is 27.7 for the caC anion and 20.5, 13.3, and 16.0 for the amino, zwitterion, and imino forms of neutral caC, respectively.21
