Abstract
Aortic sarcomas have not been linked to Lynch syndrome in humans, although other soft tissue malignancies have been. We report the case of a 31-year-old man with Lynch syndrome, who presented with abdominal pain and severe claudication. The clinical and diagnostic workup revealed near occlusion of the infrarenal aorta due to aortic angiosarcoma. En bloc resection of the visceral and infrarenal aorta with right nephrectomy was performed, facilitated by temporary extracorporeal bypass to the visceral arteries. The aorta was reconstructed with a bifurcated Dacron graft. At the 24-month follow-up examination, the patient was free of disease but was experiencing chronic diarrhea.
Keywords: Angiosarcoma, Lynch syndrome, Aortic reconstruction, Temporary bypass
We present a case of angiosarcoma of the aorta in a young man with Lynch syndrome (LS), which clinically manifested as claudication. The patient provided written informed consent for the report of his case.
Case report
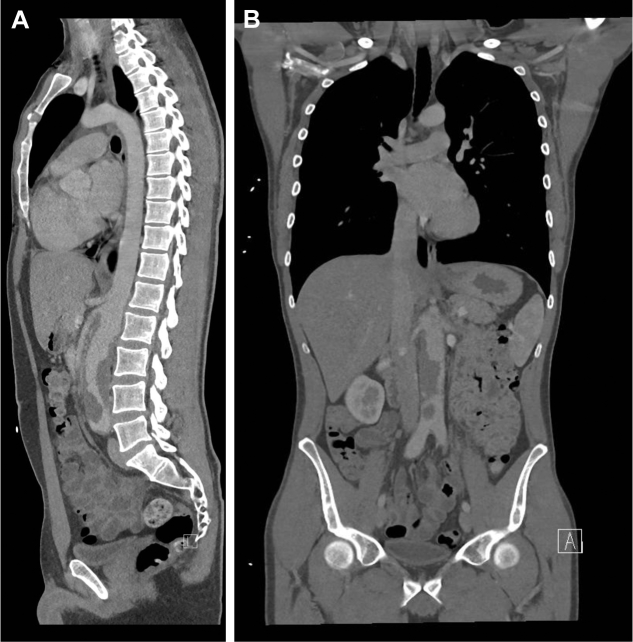
A 31-year-old white man had presented with a 12-month history of bilateral, progressive lower extremity claudication and abdominal pain. He had a diagnosis of hereditary nonpolyposis colorectal carcinoma or LS, with a mutation in the MLH1 mismatch repair gene. The findings from the initial endoscopic assessment were unremarkable. However, abdominal computed tomography (CT) imaging showed an atypical mass in the infrarenal aorta with intraluminal irregularity and near occlusion (Fig 1). Tumor or thrombus could also be seen at the origins of the superior mesenteric artery (SMA) and in the right renal artery. The mass extended distally into the common iliac arteries. Magnetic resonance angiography and CT showed occlusion of the left dorsal pedal artery but no signs of regional or distant metastases or atypical lymph nodes. A short, diffuse tissue space was present between the infrarenal aorta and the inferior vena cava. A biopsy specimen was obtained from the aortic mass through transfemoral access. The histologic examination findings were inconclusive but consistent with sarcoma.
Fig 1.
A, Sagittal and B, coronal computed tomography reconstructions of the tumor.
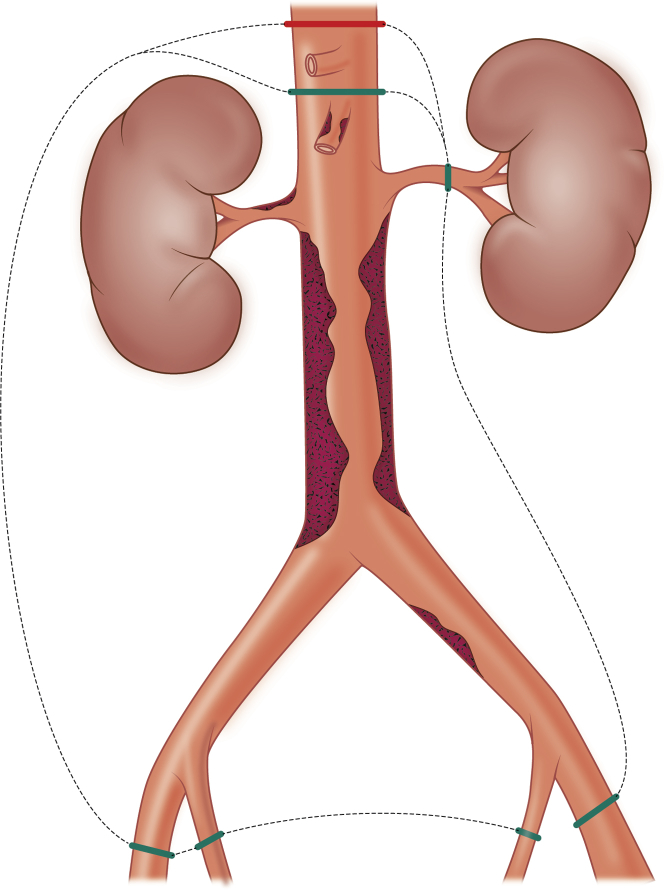
An en bloc resection of the aorta from the celiac trunk distally with possible right nephrectomy was planned. To minimize renal and visceral ischemia during aortic clamping, the plan included temporary bypass from the right axillary artery to the renal arteries and the SMA (Fig 2).
Fig 2.
The operational plan included aortic resection along the left template with possible right nephrectomy (green dashed line). Final resection shown with red dashed line.
Surgical procedure
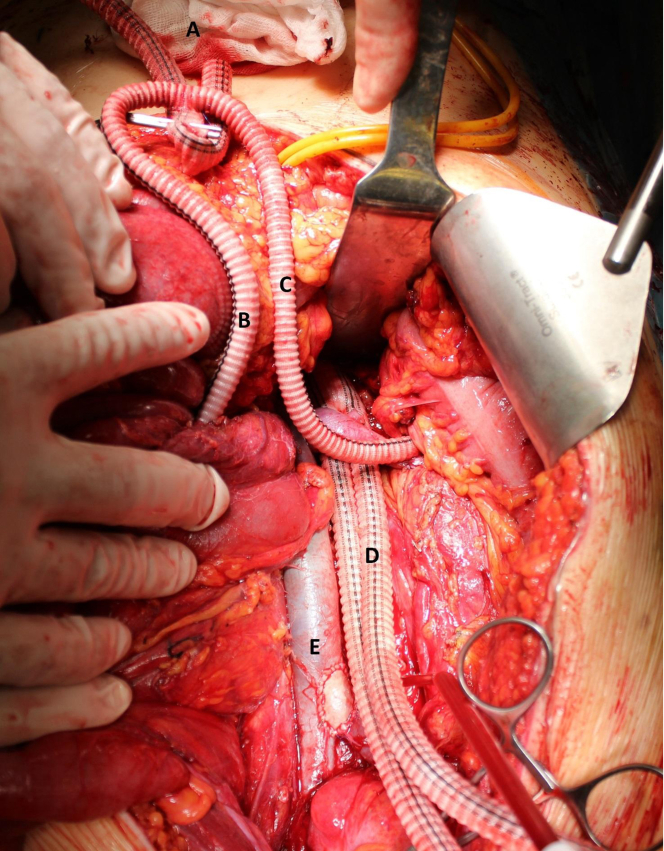
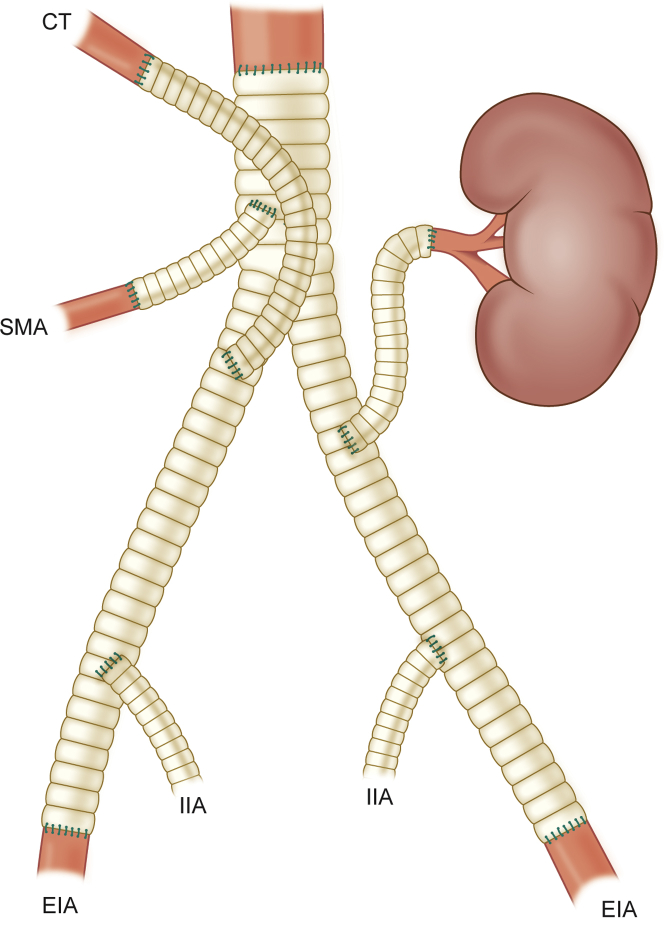
The surgery was performed 16 days after the biopsy. The aorta was approached from a midline laparotomy. Intraoperative ultrasonography was used to evaluate the tumor invasion in the arteries and ensure a tumor-free artery for anastomosis. Tumor thrombus was seen very distally in the right renal artery. An extracorporeal 8-mm Dacron graft was anastomosed to the right axillary artery. A separate 6-mm Dacron graft was anastomosed side-to-side with the 8-mm axillary graft. The ends of the 6-mm graft were then anastomosed end-to-end to the left renal artery and the SMA. The aorta was clamped below the celiac trunk. Radical en bloc tumor resection was performed, which included right nephrectomy. The aorta was resected from below the celiac trunk to the iliac arteries, including the proximal parts of both the external and the internal iliac arteries. Local resection of the inferior vena cava was performed owing to macroscopic tumor infiltration through the aortic wall and adhesion to the cava. The aorta was reconstructed with a 16/8-mm Dacron bifurcated prosthesis and the vena cava with a bovine pericardial patch (Fig 3). The aortic prosthesis was anastomosed distally to the external iliac arteries on both sides. Separate grafts were anastomosed from the main graft to the internal iliac arteries. Intraoperative frozen section samples were obtained from all arterial resection lines. All arterial margins were negative, except for the proximal aorta. Because intraoperative ultrasonography also confirmed a mass at the ostium of the celiac trunk, extension of the resection was required. Placement of a temporary bypass to the celiac artery with resection and replacement of the supraceliac aorta was performed. The temporary renal and visceral bypasses were then cut to the appropriate lengths and implanted to the aortic graft (Fig 4). The iliac and visceral arteries showed satisfactory flow rates, and distal mesenteric pulses were palpable. The bowel peristalsis was also normal. The Dacron tube was ligated at the axillary artery. The operation required 6 hours, 30 minutes. The total blood loss was 4700 mL.
Fig 3.
Intraoperative photograph showing the 8-mm Dacron axillary graft (A) and the 6-mm Dacron conduits to the superior mesenteric artery (B) and left renal artery (C). The iliac limbs (D) had not yet been anastomosed distally. The distal vena cava was reconstructed with a bovine patch (E).
Fig 4.
Illustration of the final aortic reconstruction. CT, Celiac trunk; EIA, external iliac artery; IIA, internal iliac artery; SMA, superior mesenteric artery.
Recovery and follow-up
The patient's recovery was uneventful. He spent 5 days in the intensive care unit. However, after the operation, he experienced diarrhea. The stool bacteria samples were negative for pathogens. The diarrhea was considered to be iatrogenic and due to resection of parasympathetic nerve fibers around the SMA. Aspirin 100 mg daily was started as continuous medication, augmented by subcutaneous enoxaparin 40 mg twice daily for the first 3 months.
After 3 months, the diarrhea had suddenly ceased, and the patient developed severe abdominal pains. Laparotomy was performed, and ileal strangulation caused by adhesions was found. After the repeat laparotomy, the patient had no abdominal pains or claudication but still experienced severe diarrhea and weight loss, which was managed by a dietitian. At 18 months postoperatively, he presented with World Health Organization class 0 for symptoms.
Repeated follow-up CT and magnetic resonance imaging scans of the thorax, abdomen, and leg for ≤24 months postoperatively showed no signs of tumor recurrence. The pedal artery occlusion remained. Adjuvant chemotherapy and radiation therapy were not considered to be indicated. The patient has been followed up by a sarcoma team at a university clinic.
Histologic findings
Histopathologic analysis of the resected aorta revealed poorly differentiated sarcoma in the aortic lumen. The findings were primarily consistent with epithelioid angiosarcoma. Immunohistochemistry showed CD31 and pan-cytokeratin staining, but ERG and CD117 were negative. MIB-1 proliferation in hot spots was 80%. The final resection lines and the resected inferior vena cava were free of tumor. The tumor cells were negative for MLH1, typical of LS.
Discussion
We have described a complex aortic reconstruction performed for angiosarcoma, in which temporary bypasses were placed to the visceral and left renal arteries to minimize the ischemic insult. Traditionally, the visceral and distal descending aorta have been approached from a left thoracolumbotomy. The need for en bloc right nephrectomy made this approach suboptimal. Arguably, exposure of the axillary artery would be less invasive than exposure of the descending aorta through an extended midline incision or thoracolumbotomy. The axillary approach is quite flexible if the need for further bypass emerges. The long axillary grafts can also be easily moved out of the way for visibility during aortic resection. This technique has previously been used in our institution for the repair of complicated aortic aneurysms and oncovascular tumors.1 A supraceliac trifurcated graft from midline laparotomy is an option; however, we believe it would compromise visibility and the operative circumstances during tumor resection.
LS is an autosomal, dominant disorder in the mismatch repair (MMR) genes. These include MLH1, MSH2, and MSH6. Sarcomas have been associated with cancer syndromes; however, scarce evidence of sarcoma in patients with LS has been reported.2, 3, 4, 5 Sarcomas have been associated with all the causative MMR genes.6, 7 Angiosarcoma has not been associated with LS in humans, although it has been associated with MMR mutations in zebrafish.8
Aortic angiosarcomas are rare. By 2019, 130 cases had been reported worldwide, most in small series.9, 10 Aortic sarcomas represent 90% of angiosarcomas, with differentiated tumors presenting mainly in the descending thoracic aorta and undifferentiated tumors in the abdominal aorta.11 The clinical presentation will be secondary to embolic thrombi, with ischemia of the lower extremities or visceral organs. The prognosis has been dismal, with most patients dying of tumor-related causes within 2 to 3 years. Wright et al12 proposed a clinicopathologic classification of aortic sarcomas based on the location within the vessel wall: intimal and mural. Nerve damage and subsequent diarrhea is of concern with extensive dissection of the SMA but will usually improve gradually over time.13, 14, 15
Conclusions
In selected cases, major surgery will be a feasible treatment choice for aortic sarcoma. Temporary bypasses will enable extended resection, because the issue of visceral ischemia will be eliminated. Exposure of the SMA can lead to disabling diarrhea; however, for tumor surgery, the resection must be radical, and patients should be warned of this complication.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Heinola I., Halmesmaki K., Kantonen I., Vikatmaa P., Aho P., Lepantalo M. Temporary axillorenal bypass in complex aorto-renal surgery. Ann Vasc Surg. 2016;31:239–245. doi: 10.1016/j.avsg.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Schiavi A., Lavigne J., Turcotte R., Kasprzak L., Dumas N., Chong G. Using a family history questionnaire to identify adult patients with increased genetic risk for sarcoma. Curr Oncol. 2015;22:317–325. doi: 10.3747/co.22.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilbert M., Therkildsen C., Nissen A., Akerman M., Berstein I. Sarcomas associated with hereditary nonpolyposis colorectal cancer: broad anatomical and morphological spectrum. Fam Cancer. 2009;8:209–213. doi: 10.1007/s10689-008-9230-8. [DOI] [PubMed] [Google Scholar]
- 4.Urso E., Agostini M., Pucciarelli S., Bedin C., D’angelo E., Mescoli C. Soft tissue sarcoma and the hereditary non-polyposis colorectal cancer (HNPCC) syndrome: formulation of an hypothesis. Mol Biol Rep. 2012;39:9307–9310. doi: 10.1007/s11033-012-1729-2. [DOI] [PubMed] [Google Scholar]
- 5.Carnevali I.W., Cimetti L., Sahnane N., Libera L., Cavallero A., Formenti G. Two cases of carcinosarcomas of the ovary involved in hereditary cancer syndromes. Int J Gynecol Pathol. 2017;36:64–70. doi: 10.1097/PGP.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 6.Saita C., Yamaguchi T.A., Horiguchi S.I., Yamada R., Takao M., Iijima T. Tumor development in Japanese patients with Lynch syndrome. PLoS One. 2018;13:e0195572. doi: 10.1371/journal.pone.0195572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansidhar B.J. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103–110. doi: 10.1055/s-0032-1313781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feitsma H., Kuiper R.V., Korving J., Nijman I.J., Cuppen E. Zebrafish with mutations in mismatch repair genes develop neurofibromas and other tumors. Cancer Res. 2008;68:5059–5066. doi: 10.1158/0008-5472.CAN-08-0019. [DOI] [PubMed] [Google Scholar]
- 9.Seelig M.H., Klingler P.J., Oldenburg W.A., Blackshear J.L. Angiosarcoma of the aorta: report of a case and review of the literature. J Vasc Surg. 1998;28:732–737. doi: 10.1016/s0741-5214(98)70104-1. [DOI] [PubMed] [Google Scholar]
- 10.Chiche L., Mongredien B., Brocheriou I., Kieffer E. Primary tumors of the thoracoabdominal aorta: surgical treatment of 5 patients and review of the literature. Ann Vasc Surg. 2003;17:354–364. doi: 10.1007/s10016-003-0018-x. [DOI] [PubMed] [Google Scholar]
- 11.Sebenik M., Ricci A., Jr., DiPasquale B., Mody K., Pytel P., Jee K.J. Undifferentiated intimal sarcoma of large systemic blood vessels: report of 14 cases with immunohistochemical profile and review of the literature. Am J Surg Pathol. 2005;29:1184–1193. doi: 10.1097/01.pas.0000159774.70288.7d. [DOI] [PubMed] [Google Scholar]
- 12.Wright E., Glick A., Virmani R., Page D.L. Aortic intimal sarcoma with embolic metastases. Am J Surg Pathol. 1985;9:890–897. doi: 10.1097/00000478-198512000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Nimura Y., Nagino M., Takao S., Takada T., Miyazaki K., Kawarada Y. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19:297–298. doi: 10.1007/s00534-011-0466-6. [DOI] [PubMed] [Google Scholar]
- 14.Farnell M.B., Aranha G.V., Nimura Y., Michelassi F. The role of extended lymphadenectomy for adenocarcinoma of the head of the pancreas: strength of the evidence. J Gastrointest Surg. 2008;12:651–656. doi: 10.1007/s11605-007-0451-1. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen T.C., Sohn T.A., Cameron J.L., Lillemoe K.D., Campbell K.A., Coleman J. Standard vs. radical pancreaticoduodenectomy for periampullary adenocarcinoma: a prospective, randomized trial evaluating quality of life in pancreaticoduodenectomy survivors. J Gastrointest Surg. 2003;7:1–9. doi: 10.1016/s1091-255x(02)00187-7. discussion: 9-11. [DOI] [PubMed] [Google Scholar]