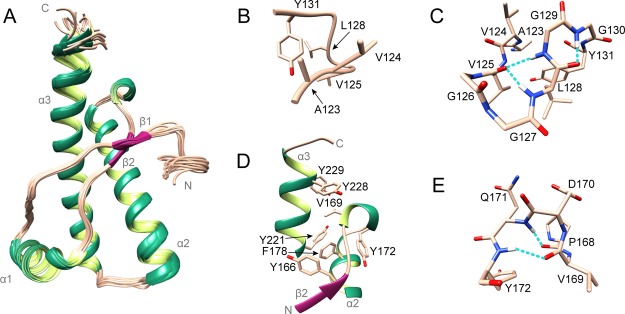
Figure 3.
Structure of mdPrP. (A) Ensemble of 20 lowest energy structures of mdPrP (residues form Ala123 to Ala233). α-Helices and 310-helix are colored green, β-sheets are colored magenta, and loops are colored champagne pink. (B) Well-defined region between residues Ala123 and Tyr131. (C) Residues from Ala123 to Tyr131 involved in the formation of α-helical turn (Val125–Leu128) and γ-turn (Leu128–Gly130). (D) Hydrophobic pocket in the proximity of the β2−α2 loop and the C-terminus of the α3 helix. (E) 310-Helix from residues Pro168 to Tyr172 inside the β2−α2 loop. Residues are presented as sticks in champagne pink and the hydrogen bonds in panels (C,E) are shown as dashed lines in cyan.