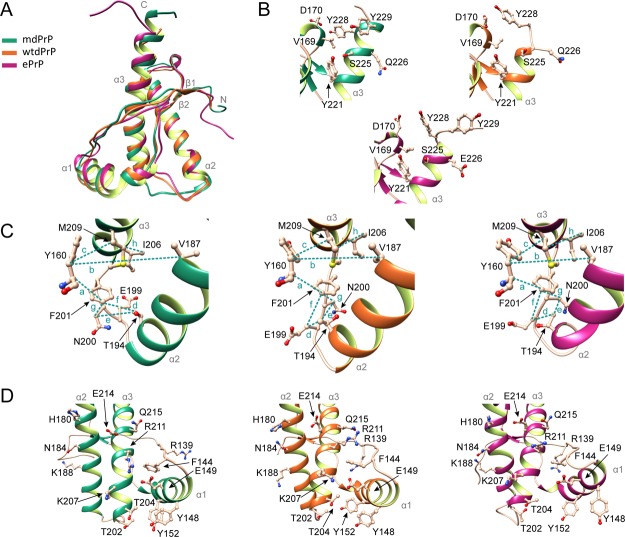
Figure 5.
Comparison of mdPrP, wtdPrP, and ePrP structures. (A) Superposition of well-defined C-terminus domains from amino acids Ala123–Ala233 of mdPrP (green), wtdPrP (orange), and ePrP (magenta). The selected residues are presented as ball-and-stick and colored in champagne pink with marked heteroatoms. (B) Structural diversity at the end of the α3 helix and the β2−α2 loop. (C) Spatial orientation of residues in the proximity of the α2−α3 loop with marked distances. Selected distances among residues are indicated with dashed lines and small letters (see Table 2 for distance information). (D) Structural differences in orientations at the α1 helix with respect to the α2−α3 V-shaped skeleton.