Figure 1.
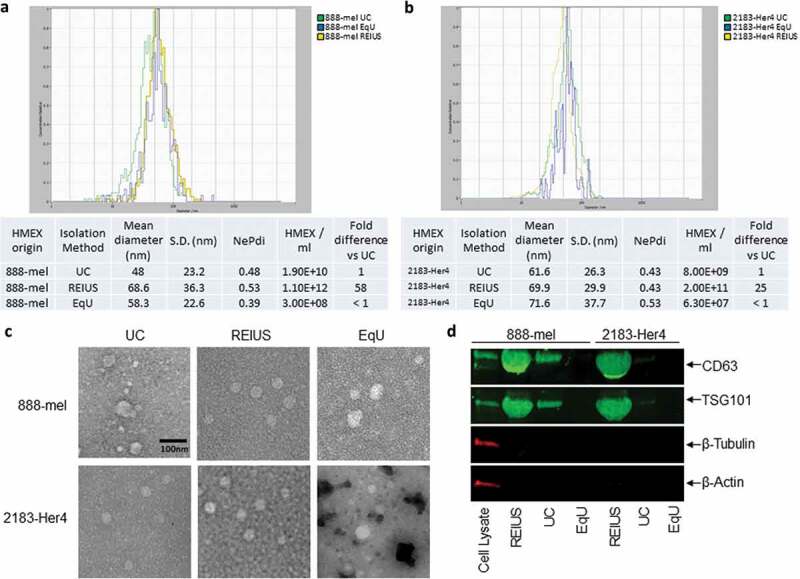
HMEX size distribution, concentration, morphology and protein characteristics in 888-mel and 2183-Her4 when various exosome isolation methods are used. 15 mL of supernatant was isolated from 16.5 × 106 cells and was processed for exosome isolation using various methods. UC: Ultracentrifugation/REIUS: Rapid Exosome Isolation using Ultrafiltration and Size Exclusion Chromatography/EqU: ExoQuick Ultra. (a) HMEX size distribution in 888-mel and (b) 2183-Her4 is consistent regardless of exosome isolation method used. Concentrations of HMEX vary depending on the method of exosome isolation chosen. Exosome polydispersity index (NePdi) is the ratio of standard deviation over mean exosome size based on NTA (Zetaview). (c) Transmission Electron Microscopy images of isolated exosomes with negative staining by Uranyless. Circular morphology and the absence of internal staining indicate intact, compartmentalized vesicles. Size is consistent with results from Zetaview. (d) Protein characteristics of exosomes using western blotting technique. Exosomes isolated using different techniques were all concentrated to a 200 µl final volume, of which 50 µl was loaded for western blotting for comparison of yield. CD63 is an exosome-enriched marker and TSG-101 is an endosomal pathway marker specific for exosomes. β-tubulin and β-actin are cytoplasmic markers. The absence of β-tubulin and β-actin compared to cell lysate sample confirms the presence of purified exosomes with minimal cytoplasmic contaminants. CD63 forms a single fused band at higher protein concentrations but forms two distinct bands when loaded at lower protein concentrations in western blot (Supplementary Figure 7).
