Abstract
Objective:
Ulcerative colitis (UC), one of the most stubborn diseases, is mainly treated by aminosalicylic acid (ASA). However, the side effects of ASA include vomiting, nausea, rash, diarrhea, headache, etc, which seriously affect life-quality of UC patients. Probiotics such as bifid triple viable (BTV) could reduce drug-induced adverse reactions and has a good clinical effect on UC. Therefore, we aimed to evaluate the clinical efficacy and safety of BTV plus ASA in treating UC.
Methods:
PubMed, Cochrane Library, Embase, Chinese Biomedical Literature Database, Chinese Scientific Journal Database, Chinese National Knowledge Infrastructure, and Wanfang databases were searched from the inception dates to October 12, 2018. Randomized controlled trials (RCTs) were included by comparing BTV plus ASA programs with ASA alone in patients with UC. Methodological quality was assessed by 2 independent researchers according to the inclusion criteria and exclusion criteria. Meta-analysis was performed by using the Review Manager 5.3 Software. Risk ratios (RRs), 95% confidence interval (CI), and standardized mean difference were calculated.
Results:
Sixty RCTs involving 4954 participants were selected for final review. Compared with ASA, BTV plus ASA significantly improved the clinical effect rate [RR = 1.23, 95% CI (1.20, 1.26), P < .00001]; reduced the relapse rate [RR = 0.34, 95% CI (0.18, 0.62), P = .0005]; and adverse effect rate [RR = 0.66, 95% CI (0.53, 0.82), P = .0002]. Compared with the controls, levels of tumor necrosis factor-α, interleukin-6 (IL-6), IL-8, C-reactive protein (CRP), hypersensitive CRP, erythrocyte sedimentation rate, and malondialdehyde were reduced; levels of IL-10, CD3+, CD4+, and superoxide dismutase were increased in BTV plus ASA group.
Conclusions:
BTV plus ASA has positive therapeutic effects on UC, and it might be a safe way to treat UC. However, comprehensive clinical trials are needed to obtain high level of clinical evidence.
Keywords: aminosalicylic acid, bifid triple viable, meta-analysis, ulcerative colitis
1. Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease of colonic mucosa. It is caused by a loss of homeostasis between intestinal immune system and gut microbiota in genetically predisposed individuals.[1] Symptoms of UC include abdominal pain, rectal bleeding, reduced stool consistency, increased stool frequency, and urgency of bowel movements.[2] Patients with UC have a high risk to get colorectal cancer.[3]
UC is associated with industrialization. As shown in epidemiological studies, incidence rates of UC vary considerably, ranging from 8.8 to 23.14 per 100,000 in North America, 0.97 to 57.9 per 100,000 in Europe, 0.19 to 6.76 per 100,000 in South America, and 0.15 to 6.5 per 100,000 in Asia.[4] Hence, the rates of UC incidence were obviously lower in developing area than the rates in developed countries. The rate of UC incidence in Asia is, however, increasing dramatically with industrialized development.[5] In urbanized areas, large-scale use of antibiotics in medicine and agriculture is common. Changes in diet and their impact on intestinal microflora during urbanization, reduced intake of carbohydrates (including natural fibers), and increased consumption of animal proteins, fats, and food additives, such as emulsifiers and artificial sweeteners, all of which can lead to a decrease in gut microbial diversity. Exposure to air pollution, which coincides with urbanization, has been shown to increase susceptibility to UC through changes in intestinal microflora. Therefore, diet, socioeconomic status, changes in hygiene status, early-life microbiota exposure, pollution, and other environmental factors have long-term effects on the human gut microbiota and influence tolerance of the host to environmental exposures, which may increase the risk of UC during urbanization.[6]
It has been largely accepted that aminosalicylic acids (ASAs) are the first-line pharmacotherapy for the treatment of UC. Adverse events caused by ASA, however, included pancreatitis, hepatotoxicity, inflammatory reactions, sexual dysfunction, cardiotoxicity, nephropathies, respiratory symptoms, and musculoskeletal complaints.[7] Patients taking ASA should be monitored for the development of new-onset organ dysfunction and UC deterioration.[7]
Bifid triple viable (BTV) has several commercial forms as capsules/powder (Bifico, Shanghai Sine Pharmaceutical, China), and enteric-coated capsules (Bifido, Jincheng Health Pharmaceutical, China). Bifico and Bifido were approved as over-the-counter drugs by State Food and Drug Administration in China, which consist of Bifidobacterium, Lactobacillus, and Enterococcus faecalis. This probiotic combination is effective in ameliorating diarrhea induced by intestinal flora disturbance or enteritis.[8] Pharmacological studies had shown that Bifico, given orally, could restore body weight, colon weight, and colon length in mice; alleviate intestinal inflammations; upregulate the level of interleukin-2 (IL-2), IL-4, and IL-10 in colonic tissues; enhance the expression of Treg cells such as CD4+, CD25+, and Foxp3+ in mesenteric lymph nodes; downregulate proinflammatory factors such as tumor necrosis factor-α (TNF-α) and Interferon-γ; and ameliorate the amount of beneficial flora and harmful flora such as Lactobacillus and Escherichia coli.[8–10] Furthermore, Bifico can improve colitis-associated cancer in mice by intervening with the possible link between Mucispirillum, Lactobacillus, Desulfovibrio, Odoribacter, and CXCR2 signaling.[10]
In recent years, more and more attention has been paid to the application of BTV plus ASA in the treatment of UC.[11–19] Clinical meta-analysis had demonstrated that, compared to mesalazine administration, mesalazine combined with bifico could increase the total effective rate of UC; raise IL-10 and superoxide dismutase (SOD) levels; restrain TNF-α, IL-8, C-reactive protein (CRP), and malondialdehyde (MDA) levels; and attenuate the clinical symptom score, endoscopic score, relapse rate, and adverse effects.[20–22] Published meta-analysis literatures of bifico,[20–22] however, had incomplete data, error data, or included with nonrandomized controlled trials (RCTs), or made no adjudgment of recognized diagnostic criteria. To provide more evidence-based advising for clinical protocols making, a meta-analysis of RCTs of BTV plus ASA program versus ASA program in the treatment of UC was conducted to assess its efficacy and safety.
2. Methods
2.1. Selection strategy
Seven major electronic databases, including PubMed, Embase, Cochrane Library, the Chinese National Knowledge Infrastructure, the Chinese Biomedical Literature Database, the Chinese Scientific Journal Database, and the Wanfang database were searched from inception to October 12, 2018, by 2 investigators (MC and ZQ) independently.
The retrieval strategy for subject words or free words was as follows: (“lived combined Bifidobacterium, Lactobacillus, and Enterococcus capsules” OR “bifid triple viable” OR “bacillus bifidus trigeminy viable-organism” OR “triple viable bifidobacterium” OR “bifidobacterium lactobacillus and enterococcus” OR “Bifico” OR “Bifido” OR “Peifeikang” OR “Beifeida”) AND (“inflammatory bowel disease” OR “ulcerative colitis”). References of retrieved literatures were checked to collect potentially relevant studies.
2.2. Inclusion and exclusion criteria
Studies were included if they met the following criteria: study type: RCTs were included; participants: all patients were diagnosed as UC[23] with no restrictions regarding ethnicity, age or sex; interventions: both the treatment and control groups received conventional therapies, on the basis of this, the treatment group was administered BTV combined with ASA including mesalazine (5-ASA), sulfasalazine (SASP) or olsalazine (OSLS), whereas the control group was orally administered ASA alone. Conventional therapies and ASA should be consistent in both groups. No limitations were set on dosages and durations of the treatment. Outcomes: one or more outcome indicators of the following should be involved: clinical efficacy, adverse effects, relapse rate, inflammation factor level, T lymphocyte subsets level, Disease Activity Index (DAI) score, endoscopic score, and lipid peroxide level.
We excluded overview, animal researches, no drug duration trials, no recognized diagnostic criteria, duplicated publications, trials with wrong data, or data missing. We only included the most recent one with the largest number of patients or longer follow-up when several trials by the same authors were identified as duplicates.
2.3. Data extraction
Two researchers (MC and ZQ) independently reviewed and extracted the following information from each study: author's name; publication year; participant number; dose of BTV, ASA, or other preparations; duration of treatment; outcomes; and adverse reactions. Any disagreements were resolved through discussion, and if necessary, arbitrated by a third reviewer (KZ).
2.4. Bias assessment
The Cochrane risk-of-bias criteria[24] was used by 2 reviewers (MC and ZQ) to assess the quality of included studies independently. The Cochrane criteria include the following items: random sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, blinding of outcome assessment, selective reporting, and other bias. Other bias was defined as trials with different baseline characteristics between different intervention groups. The researches were graded as high risk, low risk, and unclear risk.
2.5. Data analysis
Data analysis was performed using Review Manager 5.3 software. Meta-analysis to risk ratio (RR) and its 95% confidence interval (CI) of BTV plus ASA on UC for dichotomous data was performed. For continuous data, standardized mean difference (SMD) and its 95% CI were calculated.
Heterogeneity was evaluated by Q-statistic and I2 test. If the statistical heterogeneity between summary data was P < .05 or I2 > 50%, the random-effects model was used to pool the data. Otherwise, a fixed-effects model was applied (P > .05 or I2 < 50%). Sensitivity analysis of clinical efficacy was performed by the “leave-one-out” approach. If the group included >10 trials, publication bias was examined by funnel plot analysis, and Egger regression intercept was calculated using the Stata 12.0 software. Subgroup analyses were carried out based on different drug combinations, doses, and durations.
3. Results
3.1. Study identification
According to our literature retrieval strategy, 474 relevant articles were initially identified in 7 electronic databases. After excluding duplicate trials, 266 articles were selected for further analysis, and 120 articles which did not meet the inclusion criteria were excluded. A total of 146 articles were examined for the full texts and 86 were excluded. Finally, 60 RCTs met the inclusion criteria.[11–19,25–75] The flowchart of study selection is shown in Figure 1.
Figure 1.
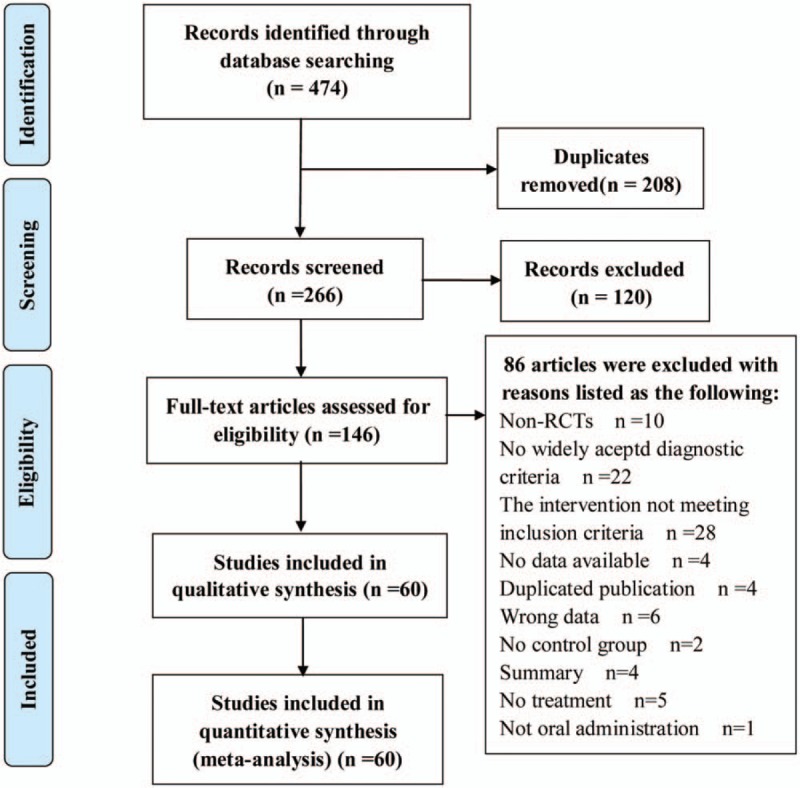
Flow diagram of study selection and identification.
3.2. Study characteristics
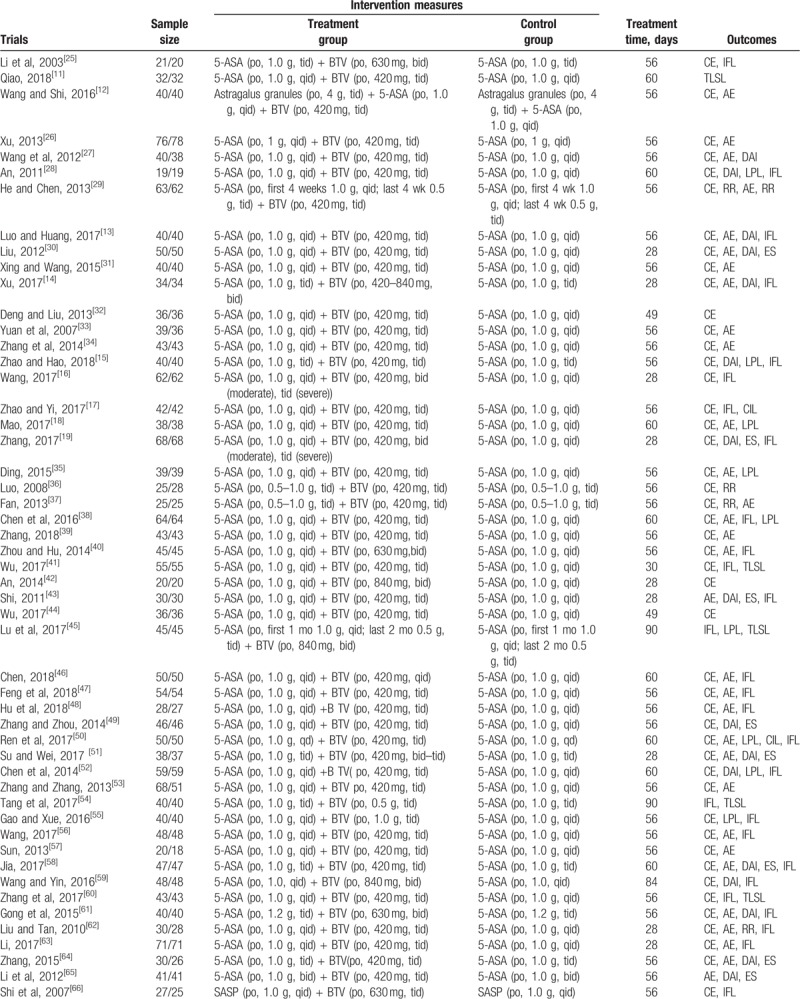
The included studies were conducted from 2003 to 2018 and involved a total of 4954 patients, with 2496 in the BTV plus ASA group and 2458 in the ASA group. All studies were performed in China with all Chinese participants involved. A 2-arm design (1 treatment group vs 1 control group) was shown. In the control group, ASA was administered as 5-ASA in 50 trials,[11–19,25–65] SASP in 7 trials,[66–72] and OSLS in 3 trials.[73–75] Patients were treated with BTV plus ASA in the treatment groups. The main characteristics of the 60 studies are summarized in Table 1 .
Table 1.
The characteristic of the eligible trials.
3.3. Risk of bias
Overall, 20 trials[13,15,17,19,28,34,38,41,46–50,52,55,58,60,63,65,70] were categorized as low risk of bias which mentioned the method of random number table, and the rest of the studies were unclear risk. One trial[25] reported number of drop-out, with 4 cases in treatment and 8 cases in control, and was assessed as low risk. Allocation concealment, blinding, selective outcome reporting, and other sources were assessed as unclear risk of bias. Figures 2 and 3 show the details of the risk of bias.
Figure 2.
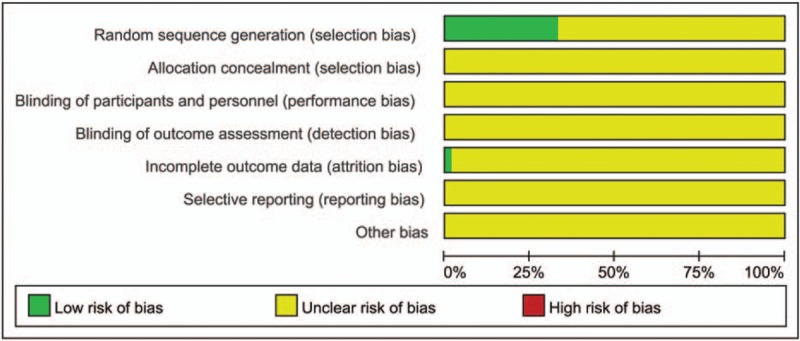
Risk of bias graph.
Figure 3.
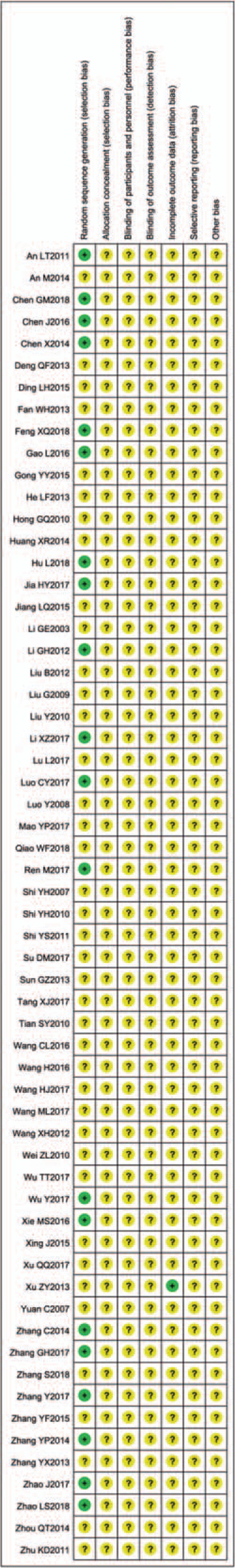
Risk of bias summary.
3.4. Clinical remission rate
Fifty-four studies[12–19,25–42,44,46–53,55–64,66,68–75] reported clinical remission in patients with UC. The meta-analysis showed that there was significant beneficial effect on the BTV plus ASA group compared with ASA using alone (RR = 1.23; 95% CI 1.20–1.26), with no significant heterogeneity between study results (P = .86, I2 = 0%). The effect estimates are shown in Figure 4.
Figure 4.
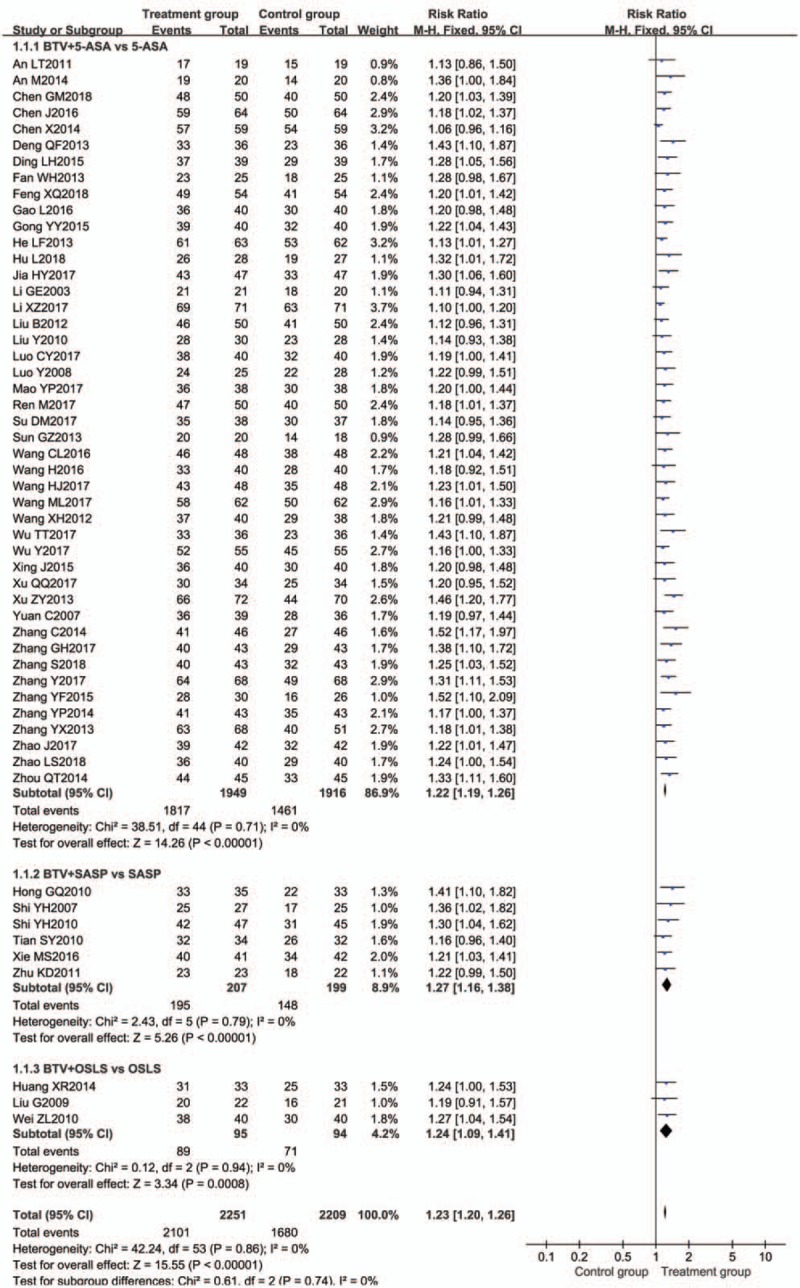
Meta-analysis of clinical remission rate of subgroup analysis of different medicine. BTV = bifid triple viable, CI = confidence interval, OSLS = olsalazine, SASP = sulfasalazine.
3.4.1. Subgroup analysis of different drug combinations
Subgroup analysis was used to evaluate the efficacy of different drug combinations. Compared with ASA alone, BTV plus 5-ASA,[12–19,25–42,44,46–53,55–64] BTV plus SASP,[66,68–72] and BTV plus OSLS[73–75] had significant improvements of clinical remission, with RR = 1.22 (95% CI = 1.19, 1.26, n = 45), RR = 1.27 (95% CI = 1.16, 1.38, n = 6), and RR = 1.24 (95% CI = 1.09, 1.41, n = 3), respectively (Fig. 4). There was no significantly different (P = .99 > .05) in the 3 ASA groups by using analysis of variance (ANOVA) of t test.
3.4.2. Subgroup analysis of different durations
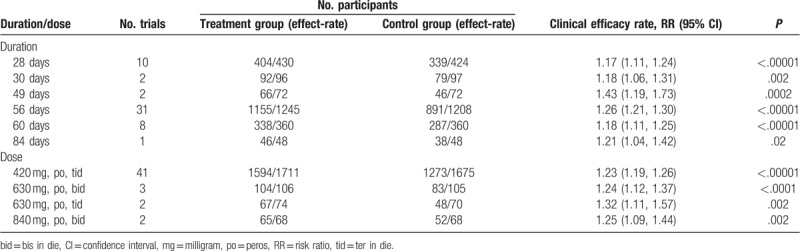
Subgroup analysis was used to evaluate the efficacy of different durations. As shown in Table 2, compared with ASA alone, BTV plus ASA durations of 28 days (n = 10),[14,16,19,30,42,51,62,63,69,71] 30 days (n = 2),[41,70] 49 days (n = 2),[32,44] 56 days (n = 31),[12,13,15,17,25–27,29,31,33–37,39,40,47–49,53,55–57,60,61,64,66,68,72,74,75] 60 days (n = 8),[18,28,38,46,50,52,58,73] and 84 days (n = 1)[59] all had significant improvements of clinical remission, with RR of 1.17, 1.18, 1.43, 1.26, 1.18, and 1.21, respectively, and P < .05 in each subgroup. The duration of 49 days showed a better remission. Notably, the small sample proportion was considered to mostly affect the accuracy of the subgroup analysis, hence, the duration of 30, 49, and 84 days was removed, and ANOVA of t test was performed in the durations of 28, 56, and 60 days, there was no significant difference among the 3 groups (P = .08).
Table 1 (Continued).
The characteristic of the eligible trials.
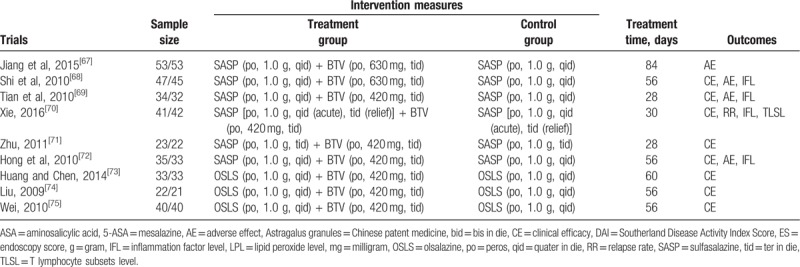
Table 2.
Subgroup analysis of association between clinical efficacy, durations, and doses.
3.4.3. Subgroup analysis of different doses
Compared with ASA alone, BTV plus ASA with doses of 420 mg [peros (po), ter in die (tid)],[12,13,15,17,18,26–39,41,44,47–50,52,53,56–58,60,62–64,69–75] 630 mg [po, bis in die (bid)],[25,40,61] 630 mg (po,tid),[66,68] and 840 mg (po,bid)[42,59] in their durations all had significant improvements of clinical remission, as shown in Table 2. According to the results, the dose of 630 mg (po,tid) had a better efficacy. The effect was enhanced with increasing daily dose, but ANOVA of t test showed that there was no statistically significant difference in efficacy between the 4 doses.
In summary, it indicates that BTV plus ASA has better potential clinical efficacy than ASA used alone. There was no significant difference between 3 drug combinations (BTV plus 5-ASA, BTV plus SASP, and BTV plus OSLS), 3 durations (28, 56, and 60 days), and 4 doses [420 mg (po, tid), 630 mg (po, bid), 630 mg (po, tid), and 840 mg (po, bid)].
3.5. Effects of BTV plus ASA on DAI
Seventeen trials[13–15,19,27,28,30,43,49,51,52,58,59,61,64–66] compared the reduction of DAI. Significant heterogeneity was found in the studies (I2 = 88%; P < .00001). Therefore, pooled RR and their corresponding 95% CIs were calculated using a random-effects model. According to result, we found that the reduction of DAI between the 2 groups was significant different [RR = −1.41, 95% CI (−1.76, −1.05), P < .00001] (Fig. 5).
Figure 5.
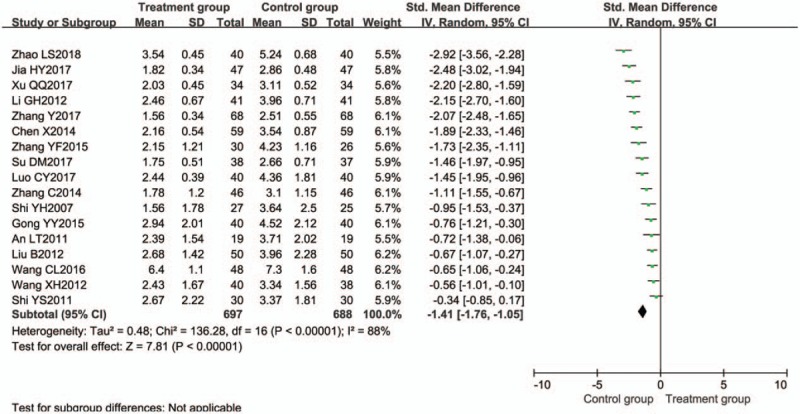
Meta-analysis of Disease Activity Index score. CI = confidence interval, SD = standard deviation.
3.6. Effects of BTV plus ASA on endoscopy score
Seven trials[19,30,43,49,51,58,64] compared the reduction of endoscopy score. Significant heterogeneity was found among studies (I2 = 85%; P < .00001). The random-effects model was applied. The pooled RR for endoscopy score was −1.13 [95% CI (−1.58, −0.68), P < .00001], which indicated that the reduction of endoscopy score between the 2 groups was significant different (Fig. 6).
Figure 6.
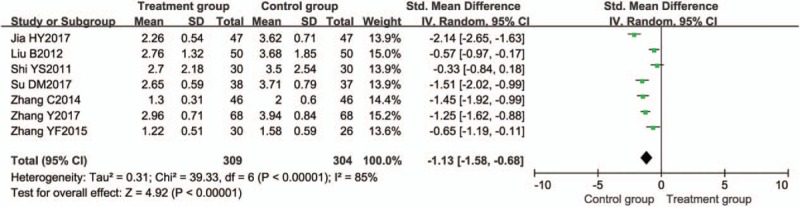
Meta-analysis of endoscopy score. CI = confidence interval, SD = standard deviation.
3.7. Effects of BTV plus ASA on inflammation factor level
Inflammation factor level was evaluated in 31 trials.[13–19,28,35,38,40,41,43,45,46,48,50,52,54–56,58–63,68–70,72] The meta-analysis results showed that the treatment groups were significantly better than the controls by decreasing the TNF-α, IL-6, IL-8, CRP, hypersensitive C-reactive protein (Hs-CRP), erythrocyte sedimentation rate (ESR), and increasing the IL-10 (Fig. 7).
Figure 7.
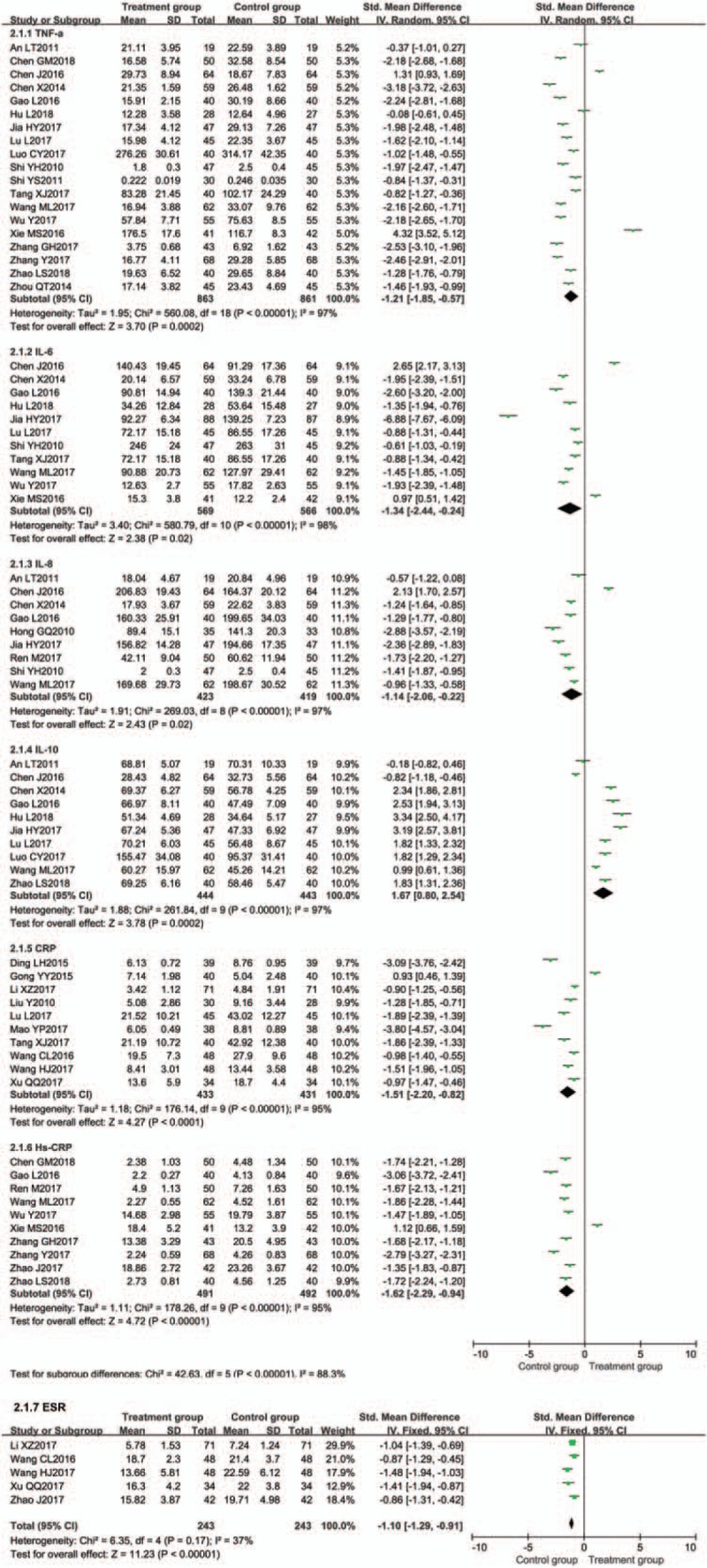
Meta-analysis of effect on inflammation factor level.
3.7.1. Tumor necrosis factor-α,
Nineteen trials[13,15,16,19,28,38,40,41,43,45,46,48,52,54,55,58,60,68,70] evaluated the expression of TNF-α between the 2 groups. TNF-α was significantly decreased in the BTV plus ASA group when compared with ASA alone [P < .00001, SMD = −1.21, 95% CI (−1.85, −0.57)] (Fig. 7).
3.7.2. Interleukin-6
Eleven trials[16,38,41,45,48,52,54,55,58,68,70] evaluated the expression of IL-6 between the 2 groups. IL-6 was significantly decreased in the BTV plus ASA group when compared with ASA alone [P = .02, SMD = −1.34, 95% CI (−2.44, −0.24)] (Fig. 7).
3.7.3. Interleukin-8
Nine trials[16,28,38,50,52,55,58,68,72] evaluated the expression of IL-8 between the 2 groups. IL-8 was significantly decreased in the BTV plus ASA group when compared with ASA alone [P = .02, SMD = −1.14, 95% CI (−2.06, −0.22)] (Fig. 7).
3.7.4. C-reactive protein
Ten trials[14,18,35,45,54,56,59,61–63] evaluated the expression of CRP between the 2 groups. CRP was significantly decreased in the BTV plus ASA group when compared with ASA alone [P < .0001, SMD = −1.51, 95% CI (−2.20, −0.82)] (Fig. 7).
3.7.5. Hypersensitive C-reactive protein
Ten trials[15–17,19,41,46,50,55,60,70] evaluated the expression of Hs-CRP between the 2 groups. Hs-CRP was significantly decreased in the BTV plus ASA group when compared with ASA alone [P < .00001, SMD = −1.62, 95% CI (−2.29, −0.94)] (Fig. 7).
3.7.6. Erythrocyte sedimentation rate
Five trials[14,17,56,59,63] evaluated the expression of ESR between the 2 groups. ESR was significantly decreased in the BTV plus ASA group when compared with ASA alone [P < .00001, SMD = −1.10, 95% CI (−1.29, −0.91)] (Fig. 7).
3.7.7. Interleukin-10
Ten trials[13,15,16,28,38,45,48,52,55,58] evaluated the expression of IL-10 between the 2 groups. IL-10 was significantly increased in the BTV plus ASA group when compared with ASA alone [P = .0002, SMD = 1.67, 95% CI (0.80, 2.54)] (Fig. 7).
3.8. Effects of BTV plus ASA on T lymphocyte subsets level
Effect on T lymphocyte subsets level was evaluated in 7 trials.[11,41,45,47,54,60,70] The meta-analysis results showed that the treatment groups were superior to the control groups regarding increasing the CD3+, CD4+, and CD4+/CD8+ (Fig. 8).
Figure 8.
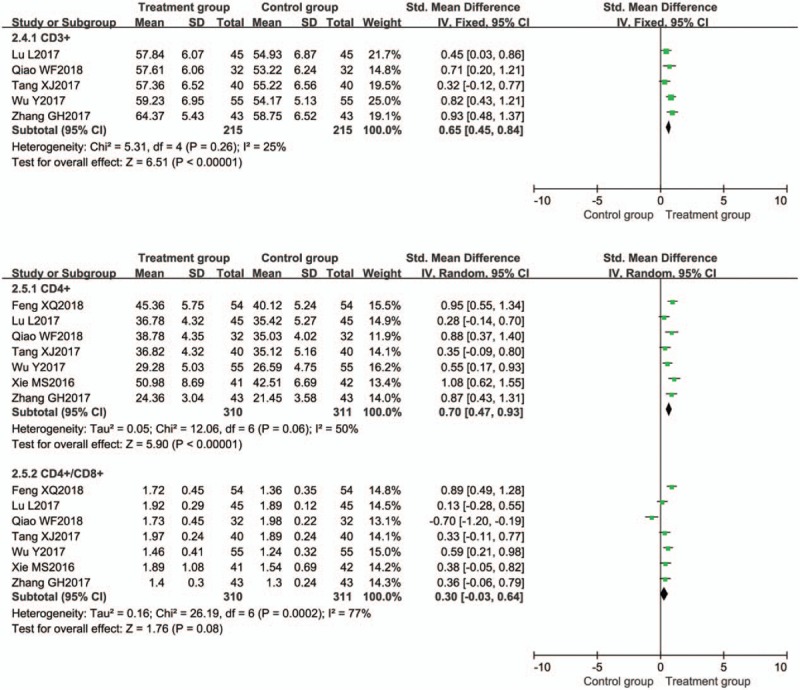
Meta-analysis of effect on T lymphocyte subsets level. CI = confidence interval, SD = standard deviation.
3.8.1. CD3+
Five trials[11,41,45,54,60] evaluated the expression of CD3+ between the 2 groups. CD3+ was significantly increased in the BTV plus ASA group when compared with control group [P < .00001, SMD = 0.65, 95% CI (0.45, 0.84)] (Fig. 8).
3.8.2. CD4+
Seven trials[11,41,45,47,54,60,70] evaluated the expression of CD4+ between the 2 groups. CD4+ was significantly increased in the BTV plus ASA group when compared with control group [P < .00001, SMD = 0.70, 95% CI (0.47, 0.93)] (Fig. 8).
3.8.3. CD4+/CD8+
Seven trials[11,41,45,47,54,60,70] evaluated the ratio of CD4+/CD8+ between the 2 groups. CD4+/CD8+ was significantly increased in the BTV plus ASA group when compared with control group [(P = .08, SMD = 0.30, 95% CI (−0.03, 0.64)] (Fig. 8).
3.9. Effects of BTV plus ASA on lipid peroxide level
Effect on lipid peroxide level was evaluated in 9 trials.[15,18,28,35,38,45,50,52,55] The meta-analysis results showed that the treatment groups were superior to the control groups regarding reducing the MDA and increasing SOD (Fig. 9).
Figure 9.
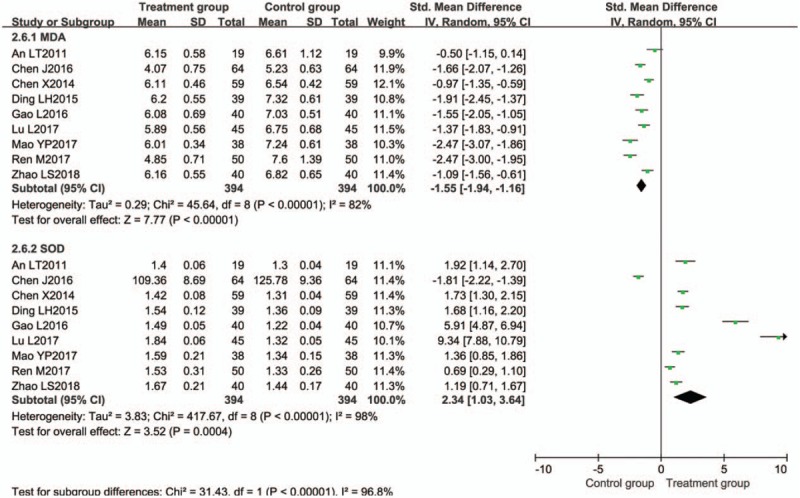
Meta-analysis of effect on lipid peroxide level. CI = confidence interval, SD = standard deviation.
3.9.1. Malondialdehyde
Nine trials[15,18,28,35,38,45,50,52,55] evaluated the expression of MDA. MDA was significantly reduced in the BTV plus ASA group when compared with control group [P < .00001, SMD = −1.55, 95% CI (−1.94, −1.16)] (Fig. 9).
3.9.2. Superoxide dismutase
Nine trials[15,18,28,35,38,45,50,52,55] evaluated the expression of SOD. SOD was significantly increased in the BTV plus ASA group when compared with control group [P = .0004, SMD = 2.34, 95% CI (1.03, 3.64)] (Fig. 9).
3.10. Relapse rate of BTV plus ASA
Five trials[29,36,37,62,70] evaluated the effect of relapse rate between the 2 groups. The relapse rate of BTV plus ASA group was 12/184, and that of control group was 36/185. We observed no significant heterogeneity (P = .98, I2 = 0%) for the relapse rate, so the fixed-effects model was used to calculate combined results. The overall estimate indicated that relapse rate in the BTV plus ASA group was significant lower than that in the control group (P = .0005), with RR of 0.34 and 95% CI (0.18, 0.62) (Fig. 10).
Figure 10.
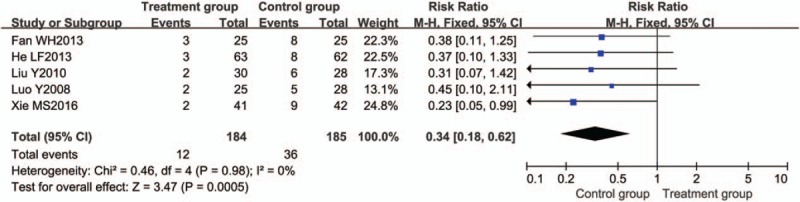
Meta-analysis of relapse rate. CI = confidence interval.
3.11. Adverse effects of BTV plus ASA
A total of 35 trials[12–14,18,26,27,29–31,33–35,37–40,43,46–48,50,51,53,56–58,61–65,67–69,72] mentioned the occurrence of adverse effects. One trial,[29] however, did not report the number of adverse effects, and 1 trial[61] reported no adverse effects. Thirty-three trials[12–14,18,26,27,30,31,33–35,37–40,43,46–48,50,51,53,56–58,62–65,67–69,72] reported adverse effects rate; adverse effects were reported in both studies, the incidence of adverse events in the BTV plus ASA group (8.3%, 118/1426) was lower than that in the control groups (12.4%, 172/1392), with a summary RR of 0.66 (95% CI 0.53–0.82; P = .62; I2 = 0%) (Fig. 11). The most common adverse events were vomiting, nausea, dry mouth, bloating, rash, dizziness, headache, arthralgia, pyrosis, and so on. It indicated that the safety profile of BTV plus ASA maybe better than ASA alone in the treatment of UC.
Figure 11.
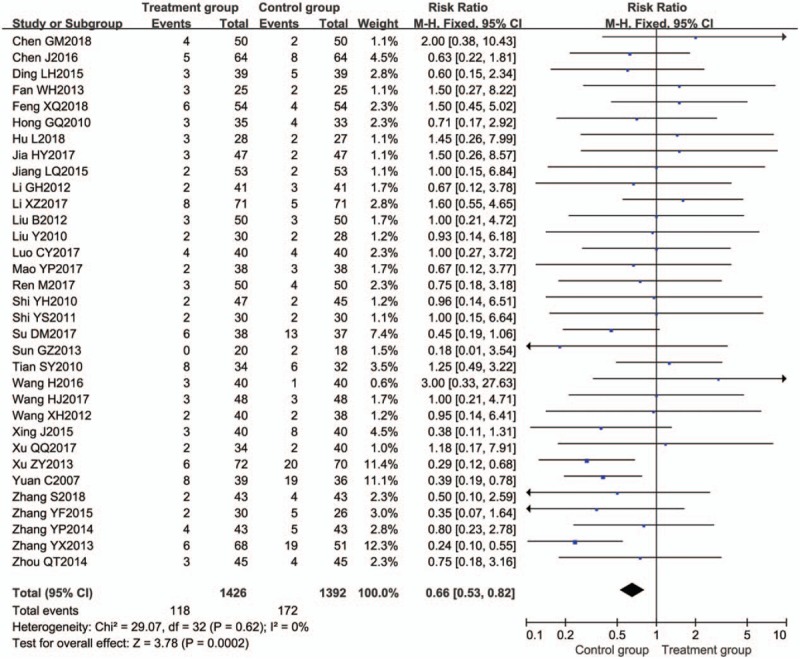
Meta-analysis of adverse reactions. CI = confidence interval.
3.12. Sensitivity analysis
Sensitivity analysis was adopted to assess the stability of the results. We used leave-one-out method by sequentially omitting each study to assess the impact of individual data on the results. Excluding any study from the clinical remission rate analysis of patients with UC did not significantly affect the results. Therefore, the meta-analysis had good reliability.
3.13. Publication bias
A forest plot of comparison of BTV plus ASA program and ASA alone for the outcome of clinical remission rates was depicted with Stata 12.0 software. As shown in Figure 12, publication bias of Egger regression showed that t = 8.32, P > |t| = .000 < .05, which revealed that there were obviously evidence of publication bias for clinical remission rates between the treatment group and the control group. The Egger publication bias plot of clinical efficiency is shown in Figure 13, and Egger's publication bias regression in Figure 14.
Figure 12.
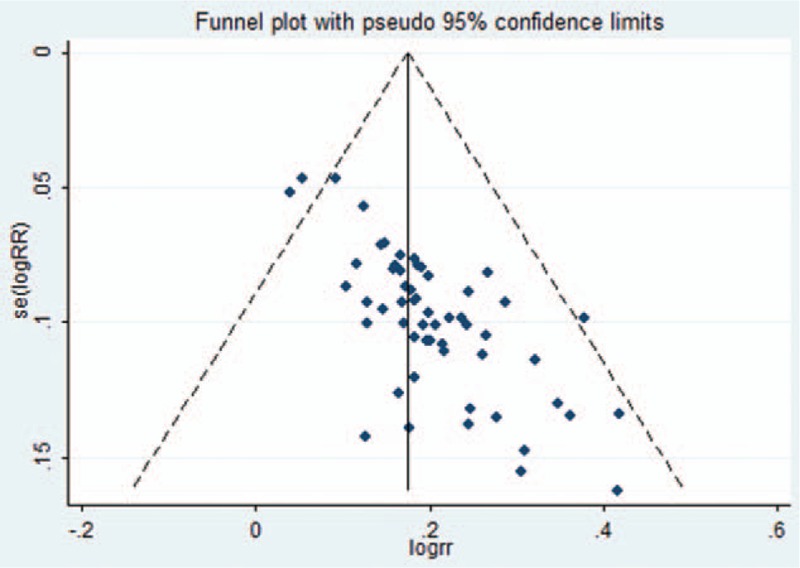
Funnel plot of clinical efficiency. RR = risk ratio.
Figure 13.
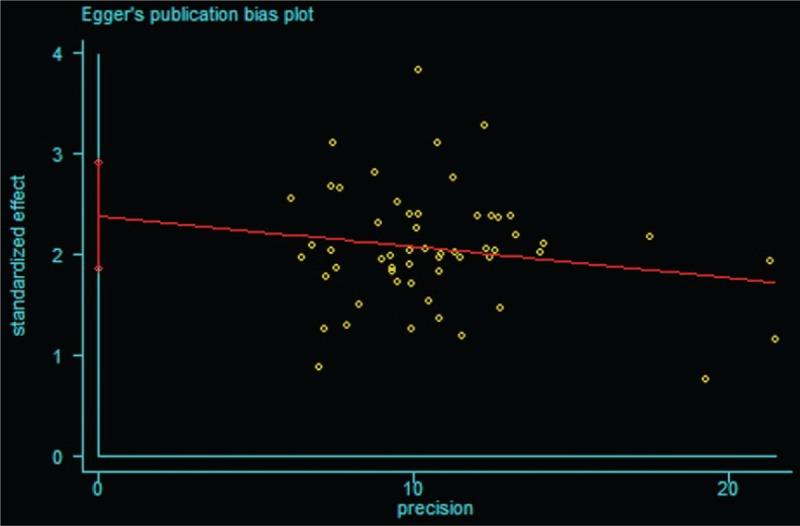
Egger publication bias plot of clinical efficiency.
Figure 14.
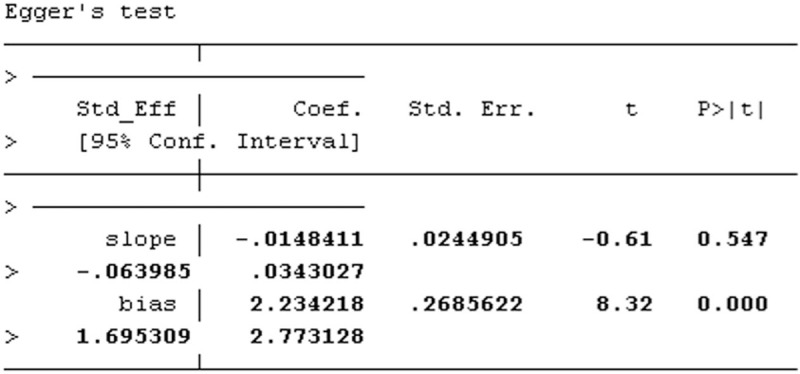
Egger publication bias regression of clinical efficiency.
4. Discussion
It has been largely accepted that the species of microbiota and its stability in patients with UC are different from normal people. The Bifidobacteriaceae family of the Actinobacteria phylum in patients with UC showed lower abundance.[76] Fecal bacteria from patients with UC had higher capacity than those in healthy patients. After fecal bacterial stimulation, the production of multiple cytokines, including TNF-α, IL-6, and IL-12, were higher in UC-active and UC-remission patients.[77,78] Notably, probiotics are associated with bacterial microbiota composition.[77] Evidence was accumulated that probiotics, such as E coli Nissle 1917, VSL3, L acidophilus, B breve, B bifidum, Saccharomyces boulardii, and so on, had a positive intervention on UC treatment, and improved the clinical efficacy of 5-ASA.[79–82]
Most meta-analysis proved that probiotics combined with mesalazine are beneficial to the treatment of UC.[80,83–85] Most studies, however, used different kinds of probiotics in treating UC, which did not provide sufficient evidence to guide the use of single probiotic. A meta-analysis of Medilac-s capsule plus mesalazine in treating UC, directly provided evidence-based testimony of 1 probiotic for clinic.[86] Since 2016, the strategy of treating UC with BTV + ASA has been increasingly recommended.[11–19,44–48,54–56,58–60,63] Therefore, to demonstrate the efficacy and safety of BTV plus ASA in UC treatment, a meta-analysis was carried out.
4.1. Summary of evidence
As shown in our results, efficacy of BTV plus ASA was 1.23 times that of ASA used alone. Subgroup of efficacy showed that there was no significant difference among 3 drug combinations (BTV plus 5-ASA, BTV plus SASP and BTV plus OSLS) and compared ASA used alone, curative remission rate changed with the duration of treatment, and the best curative effect was achieved in 49 days. But due to the smaller number of trials, it is difficult to draw firm conclusions.[87] After removing the duration of 30, 49, and 84 days with small sample proportion, this lack of an observed duration effect may be due to the small distinction between the duration of 28, 56, and 60 days. More evidence is needed to confirm the differences in different durations. Similar question had been encountered in previous published systematic reviews[87]; the effect was enhanced with increasing daily dose, and dose of 630 mg (po, tid) maybe had a better efficacy. Nonetheless, the number of the studies in doses of 630 mg (po, bid), 630 mg (po, tid), and 840 mg (po, bid) were <5; there may be too few RCTs to draw firm conclusions. More evidence is needed to confirm the differences between high and low doses. The effective dose of probiotics is influenced by many factors, including specific probiotic used, route of administration, delivery vehicle, and health endpoint. These factors make it difficult to conclude the optimal dose of probiotic.[88]
Furthermore, compared with ASA used alone, BTV plus ASA could significantly reduce the level of TNF-α, IL-6, IL-8, CRP, Hs-CRP, ESR, and MDA; significantly increase the level of IL-10, CD3+, CD4+, and SOD in patients with UC.
4.2. Safety
As shown in our results, adverse effects and relapse rate of BTV plus ASA were 0.66 times and 0.34 times lower than that of ASA used alone, respectively. It reveals that BTV plus ASA program is a safe management on UC.
4.3. Limitations
This study has several limitations should be taken into consideration. First, 60 trials stated random allocation were adopted, nevertheless, two thirds of them did not describe the method of random sequence generation. Secondly, none of the original studies made adequate descriptions of blinding and allocation concealment, which are vitally important elements to ensure methodological quality of clinical trials. The investigators and participants might have been aware of the therapeutic interventions implemented, which could lead to the emergence of false-positive conclusions. Third, only 1 out of 60 trials mentioned drop-out case, and 5 trials reported the relapse rate incidence. The results might have been different if all individuals were tested. Fourth, our meta-analysis only retrieved literatures published in English and Chinese, no reference was made to studies published in other languages, which might result in a certain degree of selective bias. In addition, 60 included trials were all conducted in China; therefore, whether the findings of our analysis could be generalized to broad ranges of regions and ethnic origin was slightly in doubt. Finally, there were subjective biases in the selection of nonquantitative outcomes, such as clinical efficacy, UC symptoms, relapse rate, adverse effects, and so on.
5. Conclusions
In this meta-analysis of RCTs, BTV plus ASA could improve clinical remission, relapse rate, adverse reactions, inflammation factor level, T lymphocyte subsets level, and lipid peroxide level in patients with UC. BTV plus ASA program can be considered to be a new approach for the treatment of UC. Nevertheless, some limitations such as potential selective bias and methodologic flaws might undermine the validity of positive findings. Further RCTs with high-quality and long-term follow-up, are recommended to generate high level of clinical evidence.
Acknowledgments
The authors thank the National Natural Science Foundation of China, Guangdong Provincial Administration of Traditional Chinese Medicine, and Guangzhou University of Chinese Medicine for their funds.
Author contributions
Conceptualization: Hui-biao Li.
Data curation: Mu-yuan Chen, Zhen-wen Qiu.
Formal analysis: Zhen-wen Qiu.
Funding acquisition: Xin-lin Chen.
Investigation: Mu-yuan Chen.
Methodology: Mu-yuan Chen, Kun-hai Zhuang.
Writing – original draft: Mu-yuan Chen, Zhen-wen Qiu.
Writing – review & editing: Mu-yuan Chen, Hong-mei Tang, Qing-qun Cai, Xin-lin Chen, Hui-biao Li.
Footnotes
Abbreviations: 5-ASA = mesalazine, ANOVA = analysis of variance, ASA = aminosalicylic acid, bid = bis in die, BTV = bifid triple viable, CI = confidence interval, CRP = C-reactive protein, DAI = Disease Activity Index, ESR = erythrocyte sedimentation rate, Hs-CRP = hypersensitive C-reactive protein, IL-6 = interleukin-6, MDA = malondialdehyde, OSLS = olsalazine, po = peros, RCT = randomized controlled trial, RR = risk ratio, SASP = sulfasalazine, SMD = standardized mean difference, SOD = superoxide dismutase, tid = ter in die, TNF-α = tumor necrosis factor-α, UC = ulcerative colitis.
How to cite this article: Chen My, Qiu Zw, Tang Hm, Zhuang Kh, Cai Qq, Chen Xl, Li Hb. Efficacy and safety of bifid triple viable plus aminosalicylic acid for the treatment of ulcerative colitis: a systematic review and meta-analysis. Medicine. 2019;98:47(e17955).
MYC, ZWQ, and HMT equally contributed to this study.
Ethics approval and consent to participate: All analyses were based on previous published studies, and thus no ethical approval and patient consent are required.
Consent for publication: All the authors declare that they agree to publish the paper in “Medicine.”
Availability of data and materials: All data supporting the findings in this study are included within the manuscript and the 2 appendix files.
This study was supported by the Traditional Chinese Medicine Bureau of Guangdong Province (No: 81774451), the Natural Science Foundation of Guangdong Province (No: 2017A030313827), the Traditional Chinese Medicine Bureau of Guangdong Province (No: 20182033), the Chuang Xin Qiang, Yuan program of First Affiliated Hospital of Guangzhou University of Chinese Medicine (No: 2017QN02).
The authors declare no conflict of interests.
References
- [1].Piscaglia AC, Lopetuso LR, Laterza L, et al. Epidemiology of inflammatory bowel disease in the Republic of San Marino: the“EPIMICI-San Marino” study. Dig Liver Dis 2019;51:218–25. [DOI] [PubMed] [Google Scholar]
- [2].Iqbal U, Anwar H, Quadri AA. Use of curcumin in achieving clinical and endoscopic remission in ulcerative colitis: a systematic review and meta-analysis. Am J Med Sci 2018;356:350–6. [DOI] [PubMed] [Google Scholar]
- [3].Xue M, Shi L, Wang W, et al. An overview of molecular profiles in ulcerative colitis-related cancer. Inflamm Bowel Dis 2018;24:1883–94. [DOI] [PubMed] [Google Scholar]
- [4].Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769–78. [DOI] [PubMed] [Google Scholar]
- [5].Vegh Z, Kurti Z, Lakatos P. The epidemiology of inflammatory bowel diseases from west to east. J Digest Dis 2017;18:92–8. [DOI] [PubMed] [Google Scholar]
- [6].Zuo T, Kamm MA, Colombel J-F, et al. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat Rev Gastro Hepat 2018;15:440–52. [DOI] [PubMed] [Google Scholar]
- [7].Sehgal P, Colombel J-F, Aboubakr A, et al. Systematic review: safety of mesalazine in ulcerative colitis. Aliment Pharm Ther 2018;47:1597–609. [DOI] [PubMed] [Google Scholar]
- [8].Shi CZ, Chen HQ, Liang Y, et al. Combined probiotic bacteria promotes intestinal epithelial barrier function in interleukin-10-gene-deficient mice. World J Gastroenterol 2014;20:4636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhao HM, Huang XY, Zuo ZQ, et al. Probiotics increase T regulatory cells and reduce severity of experimental colitis in mice. World J Gastroenterol 2013;19:742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Song H, Wang WY, Shen B, et al. Pretreatment of probiotic Bifico ameliorates colitis-associated cancer in mice: transcriptome and gut flora profiling. Cancer Sci 2018;109:666–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Qiao WF. Effect of mesalazine combined with bifid triple viable capsule on immune function and quality of life in patients with ulcerative colitis. Inner Mongolia Med J 2018;50:81–2. [Google Scholar]
- [12].Wang H, Shi YQ. Effects of Astragalus granules combined with probiotics on ulcerative colitis and its influence on IL-23 and IL-17. Chin J Ctrl Endem Dis 2016;31:1171. [Google Scholar]
- [13].Luo CY, Huang Z. Effect of mesalazine combined with Bifico on immune function and inflammatory factors of ulcerative colitis patients. Int J Dig Dis 2017;37:41–4. [Google Scholar]
- [14].Xu QQ. Clinical efficacy of mesalazine combined with Bifico in the treatment of ulcerative colitis. Tianjin Phar 2017;29:47–8. [Google Scholar]
- [15].Zhao LS, Hao SX. Effects of mesalazine combined with Bifido on lipid peroxide level and inflammation factor level of ulcerative colitis patients. Jilin Med 2018;39:136–9. [Google Scholar]
- [16].Wang ML. Effect of mesalazine combined with Bifico on ulcerative colitis and its influence on inflammatory factors. Chin Med Pharm 2017;7:43–5. [Google Scholar]
- [17].Zhao J, Yi J. Efficacy of mesalazine combined with bifid triple viable capsule in treatment of ulcerative colitis and its influence on serum Hs-CRP, ESR, PLT, and D-dimer. Medica Innovation of China 2017;14:114–7. [Google Scholar]
- [18].Mao YP. Efficacy of mesalazine combined with Bifido in the treatment of colitis. Mod Diagn Treat 2017;28:2980–1. [Google Scholar]
- [19].Zhang Y. Analysis of effect of mesalazine combined with Bifido on ulcerative colitis. Chin Mod Doctor 2017;55:43–5. [Google Scholar]
- [20].Han LC, Ye ZL, Lu XD, et al. Clinical efficacy of mesalazine combined with Bifico for treatment of ulcerative colitis: a Meta-analysis. Guangxi Med J 2017;39:1112–8. [Google Scholar]
- [21].Liao X, Yang TW. Efficacy and safety of bifidobacterium triple viable bacteria combined with mesalazine for Chinese patients with inflammatory bowel disease: a meta-analysis. J Shanxi Med Univer 2018;49:140–7. [Google Scholar]
- [22].Li H, Yang YZ, Yuan YZ, et al. Live combined bifidobacterium, lactobacillus and enterococcus capsules/powder in the treatment of ulcerative colitis: a meta-analysis. Chin J Pract Intern Med 2016;36:919–24. [Google Scholar]
- [23].Li HB, Chen MY, Qiu ZW, et al. Efficacy and safety of Kangfuxin liquid combined with aminosalicylic acid for the treatment of ulcerative colitis: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shuster JJ. Review: Cochrane handbook for systematic reviews for interventions, Version 5.1.0, published 3/2011. Julian P.T. Higgins and Sally Green, Editors. Res Synth Methods 2011;2:126–30. [Google Scholar]
- [25].Li GE, Yang AY, Xie MJ. Efficacy of Bifido in the treatment of ulcerative colitis. J Henan U Med Sci 2003;22:40–140. [Google Scholar]
- [26].Xu ZY. Clinical analysis of 154 cases of ulcerative colitis. Chinese Community Doctors 2013;15:114–5. [Google Scholar]
- [27].Wang XH, Cui LH, Pu J, et al. Analysis of therapeutic effect of combining mesalazine with Bifico in treatment of mild to moderate ulcerative colitis. Pharm J Chin PLA 2012;28:461–3. [Google Scholar]
- [28].An LT. Effect of combination of mesalazine and bifid triple viable capsules on cytokines and oxygen free radicals in patients with ulcerative colitis [Master's thesis]. Taiyuan: Shanxi Medical University; 2011 [Google Scholar]
- [29].He LF, Chen WX. Efficacy analysis of mesalazine granules combined with Bifico in the treatment of ulcerative colitis. Guide China Med 2013;11:453–4. [Google Scholar]
- [30].Liu B. Efficacy analysis of mesalazine combined with Bifico in the treatment of ulcerative colitis. World Health Digest Med 2012;9:98–9. [Google Scholar]
- [31].Xing J, Wang L. Efficacy of mesalazine combined with Bifico in treatment of ulcerative colitis. China Modern Doctor 2015;53:31–3. [Google Scholar]
- [32].Deng QF, Liu LQ. Observation of clinical effect of mesalazine combined with Bifico for patients with ulcerative colitis. China & Foreign Medical Treatment 2013;32:18–9. [Google Scholar]
- [33].Yuan C, Chen XX, Yu QL. Study of mesalazine combined with Bifico in treatment of ulcerative colitis. Modern Med Health 2007;23:2565–6. [Google Scholar]
- [34].Zhang YP, Zhou YF, Wang PF, et al. Effects of mesalazine combined with Bifico on TLR4 and MyD88 in the treatment of ulcerative colitis. J Chin Phys 2014;16:498–500. [Google Scholar]
- [35].Ding LH. Clinical observation of mesalazine combined with bifid triple viable capsules in the treatment of ulcerative colitis. J Commun Med 2015;13:42–4. [Google Scholar]
- [36].Luo Y. Study on the clinical effects of mesalazine combined with probiotics in patients with ulcerative colitis [Master's thesis]. Jinan: Shandong University; 2008. [Google Scholar]
- [37].Fan WH. Effect of combined therapy of mesalazine and probiotics in the treatment of ulcerative colitis. China Modern Med 2013;20:75–6. [Google Scholar]
- [38].Chen J, Yuan MY, Zhang XL, et al. Therapeutic effects of mesalazine combined with Bifico on ulcerative colitis and its influence on inflammation factors, stress protein and oxidative stress level. J Med Res 2016;45:57–61. [Google Scholar]
- [39].Zhang S. Analysis on the effect of Bifico combined with mesalazine in the treatment of ulcerative colitis. J Frontiers Med 2018;8:133–4. [Google Scholar]
- [40].Zhou QT, Hu JF. Efficacy of Bifico combined with different drugs for the treatment of ulcerative colitis and its impact on NF-κB and TNF-α. J Clin Res 2014;31:2364–6. [Google Scholar]
- [41].Wu Y. Effect of Bifico combined with mesalazine on inflammatory factors and immune function in patients with ulcerative colitis. J Med Forum 2017;38:138–40. [Google Scholar]
- [42].An M. Clinical study of Bifico combined with mesalazine in the treatment of ulcerative colitis. J Frontiers Med 2014;4:228–9. [Google Scholar]
- [43].Shi YS. Clinical efficacy observation of combining Bifico with mesalazine in the treatment of mide-to-moderate ulcerative colitis [Master's thesis]. Taiyuan: Shanxi Medical University; 2011. [Google Scholar]
- [44].Wu TT. Analysis of the clinical effect of mesalazine combined with Bifico in the treatment of ulcerative colitis. Health Guide 2017;272. [Google Scholar]
- [45].Lu L, Li CT, Zhang H. Effect of Bifico on inflammatory factors, oxidative stress and T lymphocyte subsets in patients with ulcerative colitis. J Hainan Med Univ 2017;23:2192–5. [Google Scholar]
- [46].Chen GM. Effect of Bifido on serum D-lactic acid and diamine oxidase levels in patients with ulcerative colitis. Med Equip 2018;31:100–1. [Google Scholar]
- [47].Feng XQ, Liang C, Liu R, et al. Effect of bifid triple viable capsules combined with mesalazine on immune function and intestinal mucosal barrier function in patients with ulcerative colitis. Int J Dig Dis 2018;38:144–7. [Google Scholar]
- [48].Hu L, Lian H, Zhou T. Effect of bifid triple viable capsules combined with mesalazine on serum inflammatory cytokines and cyclooxygenase-2 in patients with ulcerative colitis. Practical J Clin Med 2018;15:76–9. [Google Scholar]
- [49].Zhang C, Zhou Y. Evaluation of the efficacy of bifid triple viable capsules combined with mesalazine in the treatment of ulcerative colitis. J Clin Res 2014;31:696–8. [Google Scholar]
- [50].Ren M, Ye XF, Tan T. Effect of Bifico combined with mesalazine on inflammatory factors, lipid peroxidation and coagulation function in patients with ulcerative colitis. Zhejiang Clin Med J 2017;19:405–7. [Google Scholar]
- [51].Su DM, Wei XL. Analysis of the efficacy of bifid triple viable capsules combined with mesalazine granule in the treatment of ulcerative colitis. China Med Pharm 2017;7:51–4. [Google Scholar]
- [52].Chen X, Xia XZ, Sun X, et al. Efficacy of bifid triple viable capsules combined with mesalazine in treatment of patients with ulcerative colitis. Chin J Gen Pract 2014;13:223–5. [Google Scholar]
- [53].Zhang YX, Zhang XZ. Observation on the efficacy of Bifico combined with mesalazine in the treatment of mild-to-moderate ulcerative colitis. Shanxi Med J 2013;42:438–40. [Google Scholar]
- [54].Tang XJ, Wang XY, Wu GY. Effect of bifid triple viable powder on inflammatory factors and T lymphocyte subsets in patients with ulcerative colitis. J Hainan Med Univ 2017;23:1620–2. [Google Scholar]
- [55].Gao L, Xue J. Clinical effect of bifid triple viable powder combined with mesalazine enteric-coated tablets in the treatment of patients with ulcerative colitis. China Modern Med 2016;23:143–5. [Google Scholar]
- [56].Wang HJ. Effect and safety of micro-ecological agent in the adjuvant treatment of mild-to-moderate ulcerative colitis. Clin Med Eng 2017;24:499–500. [Google Scholar]
- [57].Sun GZ. Clinical observation of micro-ecological preparation combined with mesalazine in the treatment of 20 cases with ulcerative colitis. Shanghai Med Pharm J 2013;34:25–6. [Google Scholar]
- [58].Jia HY. Micro-ecological preparation combined with mesalazine in the treatment of mild-to-moderate ulcerative colitis and its influence on serum inflammatory factors. Chin J School Doctor 2017;31:601–2. [Google Scholar]
- [59].Wang CL, Yin GX. Clinical efficacy of micro-ecological agents in treatment of mild-to-moderate ulcerative colitis. World Chinese J Dige 2016;24:2731–6. [Google Scholar]
- [60].Zhang GH, Zhou H, Huang WP. Effect of micro-ecological preparation on ulcerative colitis. J Mudanjiang Med Univ 2017;38:38–40. [Google Scholar]
- [61].Gong YY, Wang YL, Sun Y. Effect of probiotics on mild-to-moderate ulcerative colitis in active stage. China Health Care Nutr 2015;25:102–3. [Google Scholar]
- [62].Liu Y, Tan RM. Clinical observation of probiotics combined with mesalazine in the treatment of ulcerative colitis. Jilin Med J 2010;31:2228–30. [Google Scholar]
- [63].Li XZ. Observation on the effect of probiotics combined with mesalazine in the treatment of ulcerative colitis. Henan Med Res 2017;26:4303–4. [Google Scholar]
- [64].Zhang YF. Clinical observation of probiotics combined with mesalazine in the treatment of mild-to-moderate ulcerative colitis [Master's thesis]. Taian: Taishan Medical College; 2015. [Google Scholar]
- [65].Li GH, Zeng S, Liao WD, et al. The effect of bifid triple viable on immune function of patients with ulcerative colitis. Gastroenterol Res Pract 2012;2012:404752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Shi YH, Huang PX, Guo CY. Study on the clinical effects of sulfasalazine combined with Bifico in patients with ulcerative colitis. J Tongji U Med Sci 2007;28:61–3. [Google Scholar]
- [67].Jiang LQ, Liu JX, Ji CJ. The effect of sulfasalazine combined with bifid triple viable capsules on mild-to-moderate ulcerative colitis. Med Inform 2015;28:265–1265. [Google Scholar]
- [68].Shi YH, Liu HG, Huang ZG, et al. Clinical effects of sulfasalazine combined with bifid triple viable capsules in patients with ulcerative colitis. Chin J New Drugs Clin Rem 2010;29:783–5. [Google Scholar]
- [69].Tian SY, Liang YF, Wei SC, et al. The effect of Bifico on serum IL-18 and IL-4 of patients with mild-to-moderate ulcerative colitis. Occup Health 2010;26:585–6. [Google Scholar]
- [70].Xie MS. Therapeutic effect of Bifico combined with sulfasalazine on T lymphocyte subsets and inflammatory factors in ulcerative colitis. Heilongjiang Med J 2016;29:668–71. [Google Scholar]
- [71].Zhu KD. Clinical observation of Bifico combined with sulfasalazine in the treatment of ulcerative colitis. Shanghai Med Pharm J 2011;32:542–3. [Google Scholar]
- [72].Hong GQ, Ye F, Ling H. Clinical observation of bifid triple viable capsules combined with sulfasalazine in the treatment of ulcerative colitis. Chin J Prim Med Pharm 2010;17:191–2. [Google Scholar]
- [73].Huang XR, Chen JN. Clinical efficacy of Bifico combined with olsalazine in the treatment of ulcerative colitis and the effect on mucosal lesions under endoscope. Pract Pharm Clin Remed 2014;17:121–3. [Google Scholar]
- [74].Liu G. Clinical effect of Bifico combined olsalazine in treating ulcerative colitis. Heilongjiang Med J 2009;33:932–3. [Google Scholar]
- [75].Wei ZL. Clinical observation of Bifico combined with olsalazine in the treatment of ulcerative colitis. China Modern Doctor 2010;48:55–6. [Google Scholar]
- [76].Moen AEF, Lindstrøm JC, Tannæs TM, et al. The prevalence and transcriptional activity of the mucosal microbiota of ulcerative colitis patients. Sci Rep 2018;8:17278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fernandes MA, Verstraete SG, Phan TG, et al. Enteric virome and bacterial microbiota in children with ulcerative colitis and Crohn's disease. J Pediatr Gastroenterol Nutr 2019;68:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang SX, Yao L, Liu YY. Fecal microbiome from patients with ulcerative colitis is potent to induce inflammatory responses. Int Immunopharmacol 2018;59:361–8. [DOI] [PubMed] [Google Scholar]
- [79].Chibbar R, Dieleman LA. Probiotics in the management of ulcerative colitis. J Clin Gastroenterol 2015;49:S50–5. [DOI] [PubMed] [Google Scholar]
- [80].Derwa Y, Gracie DJ, Hamlin PJ, et al. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharm Ther 2017;46:389–400. [DOI] [PubMed] [Google Scholar]
- [81].Wangchun SE. The mechanism of inhibition effect of probiotics on ulcerative colitis carcinogenesis and the analysis of differences in intestinal microbiota [Dissertation]. Beijing: Peking Union Medical College and Chinese Academy of Medical Sciences; 2017. [Google Scholar]
- [82].Jonkers D, Penders J, Masclee A, et al. Probiotics in the management of inflammatory bowel disease. Drugs 2012;72:803–23. [DOI] [PubMed] [Google Scholar]
- [83].Li C, Hua W, Cui GL, et al. Meta analysis of clinical effect of probiotics combined with mesalazine on inflammatory bowel disease. Henan Med Res 2017;26:3466–9. [Google Scholar]
- [84].Zhang QN, Huo LJ, Luo RL, et al. Meta analysis of effect of probiotic on remission and maintenance treatment in active ulcerative colitis. Cont Med 2016;22:4–7. [Google Scholar]
- [85].Peng LJ, Zhong Y, Wang AP, et al. Probiotics combined with aminosalicylic acid affiliates remission of ulcerative colitis: a meta-analysis of randomized controlled trial. Biosci Rep 2018;9:BSR20180943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chen MY, Li HB, Chen XL, et al. Meta-analysis of combined living Bacillus subtilis and Enterococcus faecium enteric-coated capsules plus mesalazine in treating ulcerative colitis. Chin Hosp Pharm J 2018;38:1399–422. [Google Scholar]
- [87].Zhang Y, Li L, Guo C, et al. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC Gastroenterol 2016;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ouwehand AC. A review of dose-responses of probiotics in human studies. Benef Microbes 2017;8:143–51. [DOI] [PubMed] [Google Scholar]


