Abstract
This study was aimed to genetic profiling of heat shock protein 70 (Hsp70) gene in Murrah buffalo investigating 50 unrelated adult animals at ICAR-Research Complex for Eastern Region, Patna (India) in winter, spring, and summer. PCR ready genomic DNA samples and season-wise total RNA samples were prepared. The PCR products of Hsp70 eluted from agarose gel were sequenced and analyzed. The first-strand cDNA was synthesized and concentration was equalized to 25 ng/μl. Expression kinetics of mRNA transcripts in different seasons was studied using Brilliant SYBR Green QPCR technique and the data retrieved was analyzed by least-squares ANOVA. DNA sequencing by primer walking revealed four allelic variants of Hsp70 gene. Alignment study revealed one substitution in 5′UTR, six substitutions in coding region, and one addition in 3′UTR. The highest percent identity and negligible phylogenetic distance were found among the alleles and reference bovine sequences. The relative mRNA expression was significantly higher in summer when THI ≥ 84 than the spring and winter; fold change increased by 4.5 times in summer than the spring whereas found nearly half in winter. These findings can be useful for heat stress management in buffaloes and help in understanding the mechanism of thermo-regulation well.
Electronic supplementary material
The online version of this article (10.1007/s12192-019-01042-7) contains supplementary material, which is available to authorized users.
Keywords: Hsp70 gene, mRNA expression, Murrah buffalo, Polymorphism, SNP
Introduction
Murrah buffalo is one of the best milch breed in India as well as in the world and is well known for its resistance to tropical diseases, low cost of maintenance, and has higher percentage of milk constituents (Thiruvenkadan et al. 2013). On the sub-tropical region of India, temperature in the summer rises about 40−48 °C which unquestionably is out of the comfort range of animals and causes stress. Thermal stress redistributes the body resources including protein and energy at the cost of decreased growth, reproduction, production, and health (Mallick 2015), and physiological mechanisms including endocrinal and cellular responses are triggered to maintain homeostasis. At cellular level, the ability to survive and adapt to thermal stress involves biochemical responses and gene expression mediated by a family of highly conserved proteins named heat shock proteins (HSPs) which is induced by elevated temperatures or a variety of cellular stresses (Ross et al. 2003). Induced expression of HSPs corrects protein misfolding caused by thermal stress at cellular level (Basirico et al. 2011). HSP expression acts as a potential indicator of animal adaptation to harsh environmental stress (Hansen 2004). While stress-induced synthesis of HSPs represents a generalized molecular mechanism displayed by almost all cells, individual animals differ in their capacity to manage with stress. There exists a strong correlation between the induction of HSPs and the induction of thermotolerance by preventing activation of stress kinases (Gabai et al. 1997). These proteins were extensively studied earlier and purified in most of the livestock species and different breeds of bovines (Lacetera et al. 2006; Kristensen et al. 2004). The 70 kDa HSP family (Hsp70) has been categorized into constitutive and inducible forms, which contribute to stress tolerance by escalating the chaperone activity in the cytoplasm (Leppa and Sistonen 1997). The heat shock response is a rapid and transient gene-expression program (Richter et al. 2010). The inducible Hsp gene expression is regulated by the heat shock transcription factors (HSFs) (Akerfelt et al. 2007). HSFs present in the cytosol are bound by HSPs and maintained in an inactive state, where stressors activate HSFs, causing them to separate from HSPs. HSFs are phosphorylated by protein kinases and translocate into nucleus to form HSF-trimer complexes which bind to heat shock elements (HSE) in the promoter region of the Hsp gene. Hsp mRNA is then transcribed and leaves the nucleus for the cytosol, where new HSP is synthesized (Mallick 2015). Mammalian HSF1 is required for inducible Hsp expression and acquired thermotolerance, a function that is not compensated for by other members of the HSF family (McMillan et al. 1998). As individual animals differ in their capacity to manage with stress, we speculated that there was genetic variability in heat shock protein genes among the individuals, and as the induction of thermotolerance has strong correlation with induction of HSPs, expression of these genes would be elevated to manage summer stress. Hence, the present study was aimed to genetic profiling of heat shock protein 70 gene (Hsp70) in Murrah buffalo under sub-tropical climate of India investigating polymorphism of Hsp70 gene and its expression pattern in summer, winter, and spring seasons.
Material and methods
Experimental sampling procedure
Investigation was carried out on 50 unrelated adult females (46 to 76 months aged in first to fourth lactating stage) of Murrah buffalo maintained with ad libitum access to feed and water, and standard management at the organized dairy farm of ICAR-Research Complex for Eastern Region, Patna (India). The ambient temperature (minimum to maximum) prevailing entire experimental period was 10.24 ± 1.57 °C to 22.81 ± 1.42 °C in winter (mid November to mid February), 23.65 ± 3.46 °C to 30.37 ± 2.36 °C in spring (mid February to mid May), and 28.82 ± 1.32 °C to 39.17 ± 2.38 °C in summer (mid May to mid August). The thermo-humidity index (THI) was calculated based on formula developed by National Research Council 1971: THI = (Tdb + Twb) × 0.72 + 40.6, where Tdb and Twb are the corresponding dry bulb temperature and wet bulb temperature in degree Celsius. For genomic DNA extraction, about 5 ml of blood was collected from jugular vein of each animal in a sterile polypropylene vial containing 2.7% EDTA (0.25 ml) as an anti-coagulant under sterile condition. For RNA extraction, 5 ml blood was collected in a DEPC (0.1% diethyl pyrocarbonate)-treated sterile vial from jugular vein of each animal in three different seasons namely winter, spring, and summer at 15 days interval. The blood samples were transported to the laboratory in an icebox and stored at − 20 °C till further processing.
Genomic DNA sample preparation
Genomic DNA was isolated from the blood samples following phenol/chloroform extraction procedure (Kagami et al. 1990). DNA pellet in the Eppendorf tubes was air-dried for 1 h to remove traces of ethanol, then dissolved in 200-μl TE buffer, and incubated in water bath at 60 °C for 2 h to inhibit DNAse activity and to dissolve pellet properly. Finally, the DNA was cooled and stored at − 20 °C for further use. DNA purity was checked using UV-spectrophotometry, and the samples showing OD ratio (260 nm/280 nm) between 1.7 and 1.9 were considered good and used for PCR amplification. DNA concentration (μg/μl) was determined as (OD260 × dilution factor × 50)/1000 as 1 OD value at 260 nm is equivalent to 50 ng dsDNA/μl. DNA quality was checked by horizontal submarine 0.8% (w/v) agarose gel electrophoresis. Only DNA samples showing intact band and devoid of smearing were used for further analysis. The PCR ready DNA samples were prepared at a concentration of 80 ng/μl.
Primers and PCR amplification
The primer was designed for amplification of Hsp70 gene from the published genomic DNA sequence of homologues species (Sodhi et al. 2013) using LASERGENE software (Version 1.83) (DNASTAR, Inc., Madison WI, USA) and synthesized from GCC Biotech (India) Pvt. Ltd. (Kolkata). The annealing temperature (Ta) of each primer pair was optimized following standard protocol (Wimmers et al. 2000) using Gradient PCR (Eppendorf gradient, Germany).
The PCR reactions were carried out in 25-μl reaction mix prepared by gently mixing 1.0 U of Platinum™ 2X Hot Start PCR master mix (Thermo Fisher Scientific Inc., USA) (12.5 μl), 10 pM of each primer (1 μl), and 80 ng of template DNA (1 μl) into nuclease-free water (9.5 μl). The PCR amplification was carried out in a programmable thermal cycler (PTC-200, MJ Research, USA) following the thermal cycles of the initial denaturing at 95 °C for 5 min, followed by 35 cycles of (i) denaturation at 95 °C for 30 s, (ii) annealing at 64 °C for 60 s, and (iii) extension at 72 °C for 150 s, followed by a final extension at 72 °C for 10 min and then 4 °C forever.
PCR product analysis and sequencing
The probable molecular sizes of the amplified products were determined through horizontal submarine 1.5% (w/v) agarose (low EEO) gel (in 0.5× TBE buffer) electrophoresis. Approximately 5 μl of the PCR product mixed with 1 μl of 6× gel loading dye (bromophenol blue and xylene cyanol) was loaded along with 1 μl of Lamda DNA/HindIII Marker (Thermo Fisher Scientific Inc., USA) in a separate lane and run at 60 V (7 V/cm) for 2 h. Then, the products onto the gel were observed under UV transilluminator and documented by gel documentation system (Biorad, USA). PCR product from agarose gel electrophoresis was extracted using Sure extract PCR clean up/gel extraction kit (Genetix Biotech Asia Pvt. Ltd., New Delhi) as per manufacturer’s protocol. Amplified product was sequenced by Primer Walking using Sanger Sequencing in an Automatic ABI Prism DNA Sequencer from Eurofins Genomic India Pvt. Ltd. (Bangalore).
Total RNA sample preparation
Total RNA samples were extracted from whole blood (5 ml in a DEPC-treated sterile vial) of each animal in three different seasons namely winter, spring, and summer using Nucleopore GRNA Blood Kit (Genetix Biotech Asia Pvt. Ltd., New Delhi) as per manufacturer’s protocol and dissolved in 50 μl of DEPC-treated nuclease-free water. The purity and quantity of the extracted RNA were determined by UV-spectrophotometry at 260 nm/280 nm. Samples showing absorbance ratio (260 nm/280 nm) in between 1.9 and 2.2 were considered for further analysis and as 1 absorbance unit at 260 nm equates to 40 μg/ml of RNA, the sample concentration (μg/ml) = OD260 × 40 was computed and adjusted to make a uniform concentration (1000 ng/μl) and stored at − 20 °C till further use. Quality and integrity of RNA were checked using 2.2 M formaldehyde denatured 1% agarose gel electrophoresis (Sambrook and Russell 2001) using 1% agarose in DEPC-treated water. The RNA samples were prepared by mixing 2 μl RNA with 2 μl of 10× gel running buffer (MOPS), 4.0 μl of formaldehyde, and 10 μl of formamide and kept at 65 °C for 15 min thereafter immediately chilled on ice for 5 min. After a brief pre run of the gel for 5 min at 5 V/cm, the samples were loaded into the wells and run in 1× MOPS gel running buffer for 2 h and viewed under UV-transilluminator. Good quality RNA showed two intact bands (18S rRNA and 28S rRNA), the mRNA appeared as a smear spanning between 28S and 18S rRNA. There was no smear lower to the 18S rRNA band which indicated good quality mRNA. The absence of band near the well indicated the purity of RNA sample free from the genomic DNA contamination.
First-strand cDNA synthesis
The first-strand cDNA was prepared from 1 μl of purified mRNA sample using Genesure first-strand cDNA synthesis kit (Genetix Biotech Asia Pvt. Ltd., New Delhi) with oligo dT primer as per the protocol provided with the kit. Positive and negative control reaction was used to verify the results of the first-strand cDNA synthesis steps: reverse transcriptase minus (RT−), negative control to assess any genomic DNA contamination of the RNA sample, no template negative control (NTC) to assess any reagent contamination, and positive control RNA template (GAPDH RNA: 50 ng/μl) with GAPDH gene-specific primers. The quality and quantity of the first-strand cDNA were checked by UV-spectrophotometry, and the samples having OD260/OD280 ratio in the range of 1.7 to 1.8 were considered as good cDNA samples. The first-strand cDNA product was directly used in PCR applications or stored at − 20 °C for less than 1 week. Concentration of cDNA of each sample was equalized to 25 ng/μl for use in real time quantitative reverse transcription PCR (qRT-PCR) for gene expression assays and stored at ultra low (− 80 °C) temperature until further use.
Quantitative real-time PCR analysis
Expression profile of mRNA transcripts of Hsp70 gene in different season (corresponding to 21, 26, and 28 nos. of mRMA transcripts in winter, spring, and summer) in Murrah buffalo was analyzed by real-time quantitative reverse transcription PCR (qRT-PCR) using Brilliant® SYBR® Green QPCR technique (QIAGEN India Pvt. Ltd., New Delhi) according to the manufacturer’s instructions. For real-time PCR amplification, two pairs of primer having similar annealing temperature as well as product size were designed and synthesized from GCC Biotech (India) Pvt. Ltd. (Kolkata) for amplification of Hsp70 gene and internal control gene (GAPDH) from the published genomic DNA sequence of homologue species using LASERGENE software and used (Table 1). The PCR conditions for each target gene were optimized, and the optimum annealing temperatures are presented in Table 1. Brilliant® SYBR® Green QPCR master mix (QIAGEN India Pvt. Ltd., New Delhi) was used for the preparation of the PCR reaction mixture. All the reactions in nuclease-free 8-tube strips with optically clear flat caps (Axygen Scientific Inc., USA) were run in STRATAGENE Q-CYCLER using MX 3000P software. Each sample was run in duplicate both for target gene (Hsp70) as well as internal control (GAPDH). Especially, the primer concentration was standardized to prevent the primer-dimer formation, which may result in false fluorescence, i.e., picked by Q-CYCLER which reduces the range of the linear relationship between the number of cycles needed to detect the PCR product and the initial template amount. The standardized concentrations of the components used in 20-μl reaction mixture were 10.0-μl 2× SYBR® Green master mix, 0.75 μl of each forward and reverse primer (0.19 μM), 1 μl cDNA template, and 7.5 μl nuclease-free water. A negative control containing all the reaction components except the cDNA template in triplicate was also set up to check any contamination of foreign DNA in the reaction components. Brilliant® SYBR® Green QPCR master mix contains hot start Taq DNA polymerase which requires initial activation at 95 °C and so one extra step of initial activation is required for real-time PCR in comparison to normal PCR. Several combinations of programs were tried before setting up the program giving the best amplification of both the Hsp70 gene and GAPDH. The thermal profile consists of holding stage I at 50 °C for 2 min, holding stage II at 95 °C for 5 min followed by PCR 40 cycles of denaturation at 95 °C for 30 s, annealing at optimized temperature (61.5 °C) for 30 s and extension at 72 °C for 30 s; followed by detection of fluorescent signal by the real-time detection system to generate amplification curve. Each sample was subjected to 60–95 °C at + 0.5 °C increment for 10 s to generate dissociation curve or melt curve to ensure specific amplification. Then threshold cycle (Ct) values and melting point temperature of each tube were retrieved and reviewed for its corresponding amplification and dissociation curve. The actual raw data (Ct) from qRT-PCR experiment was imported in excel and average Ct values were derived for each sample for target and internal control gene.
Table 1.
The PCR primers for Hsp70 gene amplification and its mRNA expression along with their sequences, optimized annealing temperatures (Ta), and expected product sizes in base pairs (bp)
| Gene | Primers | Oligo nucleotide Sequence | Ta (°C) | Exp. Product size (bp) | Reference |
|---|---|---|---|---|---|
| Gene amplification | |||||
| Hsp70 | HspF / HspR | 5′ACTGAACTCGGTCATTGGCT3′ / 5′AGAGGCCAATTGCAGTTCAT3′ | 64.0 | 2423 | Sodhi et al. 2013 |
| mRNA expression | |||||
| Hsp70 | P1 / P2 | 5′CTCGTCGATGGTGCTGACCAAG3′ / 5′TCCTGTCCAGGCCGTAGGCGAT3′ | 61.5 | 206 | NM_174550.1 |
| GAPDH | P3 / P4 | 5′AGGTCATCCCTGAGCTCAACGG3′ / 5′TCGCAGGAGACAACCTGGTCCTCA3′ | 61.5 | 199 | NM_001034034.2 |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase mRNA
Quantification of mRNA expression
The mean Ct values of the test gene were normalized with internal control gene to generate effective raw data and expressed as the relative expression of Hsp70 mRNA to the internal control gene (∆Ct) for each sample under each season, and also, the relative expression of the Hsp70 mRNA in a specific season with respect to the reference season was transformed in terms of fold change (log-normal with geometric mean) following the double delta Ct method (Livak and Schmittgen 2001).
In silico and statistical analysis
Alignment, homology, and phylogenetic study were carried out for nucleotide sequences of various allelic variants by using MegAlign and ClustalW tools of LASERGENE software (Version 1.83) (DNASTAR, Inc, Madison WI, USA).
The ∆Ct data generated so far in three different seasons were analyzed by least squares analysis of variance (LS ANOVA) using JMP 9.1 statistical program package of SAS (2008) incorporating season as fixed effect in the model: Yij = μ + Si + eij, where Yij, value of ∆Ct in ith season on jth individual; μ, overall mean; Si, fixed effect of ith season; and eij, random error of ∆Ct values of jth individual under ith season associated with mean ‘0’ and variance σ2. The mean ∆Ct values were compared using Tukey’s HSD test (Tukey 1953).
Results
Genetic variability of Murrah Hsp70 gene and homology
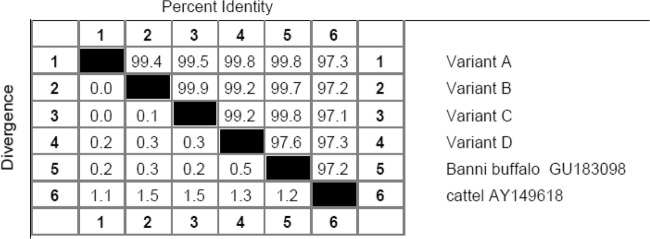
The PCR-amplified products of Hsp70 gene in Murrah buffalo samples when run onto the agarose gel electrophoresis represented 2561 bp as product length (Fig. 1) and DNA sequencing by primer walking revealed four allelic variants designated as allele A (2511 bp), B (2514 bp), C (2509 bp), and D (2283 bp). The NCBI GenBank Accession numbers of these alleles and the allelic differences are presented in Table 2. The allelic sequence alignment study (Figs. S1 and S2; Supplementary material) revealed single-nucleotide differences at eight base positions among the alleles, out of which one substitution was found in the 5′UTR, six substitutions in the coding region, and one addition in the 3′UTR. B allele differed from A allele by having one silent mutation in the coding region (guanine instead of thymine) at 1043th base position. C allele differed from A allele by substitution of guanine into cytosine at 75th base position in the 5′UTR. D allele differed from A allele by having two silent mutations at coding region where adenine was replaced by guanine at the 1910th base position and guanine replaced by adenine at the 1949th base position. D allele further differed from A allele by having three functional mutations in the coding region at 1908th, 2071th, and 2123th base positions of nucleotide sequence where guanine was replaced by adenine at 1908th and 2071th position, and guanine replaced by cytosine at 2123th base position. This led to change in amino acid sequence in D variant where glutamic acid was replaced by lysine, serine replaced by asperagine, and glutamine replaced by histidine at corresponding 554th, 608th, and 625th position of amino acid sequence (Table 2). Homology study (Fig. 2) showed the highest percent identity of the nucleotide sequences among the allelic variants of Hsp70 in Murrah buffalo, and these sequences when compared with the reference sequences in cattle and Banni buffalo (Fig. 2) showed also the highest percent identity. Phylogenetic study (Fig. 3) revealed the evolutionary relationship among the allelic variants of Hsp70 gene where allele B and C formed a cluster indicating their close evolutionary relationship, while allele A was phylogenetically distant from B and C cluster, and D allele was also more distant from A, B, and C cluster. Furthermore, these sequences when compared with the reference sequences in cattle and Banni buffalo (Fig. 2) showed that the sequences of buffalo allelic variants were in closer cluster than the cattle sequence.
Fig. 1.

Submarine horizontal 1.5% (w/v) AGE representing PCR-amplified products of Hsp70 gene in Murrah Buffalo
Table 2.
The allelic variants of Hsp70 gene in Murrah buffalo along with their molecular sizes in base pair (bp), NCBI GenBank Accession nos., single-nucleotide substitution, and deduced change in amino acid sequences
| Allelic variants | Allele size (bp) | NCBI GenBank Accession nos. | Base position of single-nucleotide substitution | Deduced change in amino acid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 75 | 1043 | 1908 | 1910 | 1949 | 2071 | 2123 | 2513 | 554 | 608 | 625 | |||
| A | 2511 | MH814759 | G | T | G | A | G | G | G | X | Glu | Ser | Gln |
| B | 2514 | MH814760 | G | G | G | A | G | G | G | T | Glu | Ser | Gln |
| C | 2509 | MH814761 | C | T | G | A | G | G | G | - | Glu | Ser | Gln |
| D | 2283 | MH814762 | G | T | A | G | A | A | C | X | Lys | Asn | His |
Fig. 2.
Homology based on nucleotide sequences of allelic variants of Hsp70 gene in Murrah buffalo and other bovines
Fig. 3.
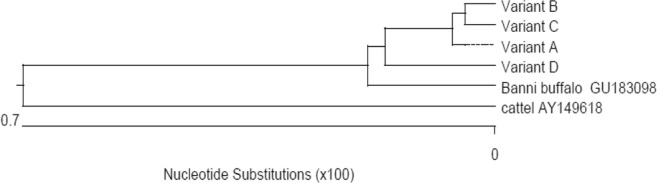
Phylogenetic tree based on nucleotide sequences of allelic variants of Hsp70 gene in Murrah buffalo and other bovines
Hsp70 mRNA expression in different seasons
The thermo-humidity index (THI) was calculated as 53.86 ± 4.38, 68.56 ± 6.94, and 84.74 ± 7.32 in winter, spring, and summer, respectively, as represented in Fig. 4, and the mRNA expression of Hsp70 gene in Murrah buffalo was studied through the kinetics of RT-qPCR in different seasons prevailing with these calculated THI. The real-time qPCR of each sample showed clear amplification plot (Figs. S3, S4; Supplementary material) and dissociation curve (Fig. S5; Supplementary data) without any primer dimer formation, and its multi-component plot (Fig. S6; Supplementary material) was examined to ensure that the reaction was set up correctly and the reaction mix had not evaporated during the approximately 90-min-long PCR amplification. Lack of amplification in the negative control as found non-increased curves corroborates the validity of the test. The least squares means of ∆Ct values of mRNA expression of Hsp70 gene in Murrah buffalo were estimated 7.91 ± 1.51, 6.76 ± 2.16, and 4.59 ± 1.62 in winter, spring, and summer seasons, respectively. The ∆Ct values and fold change of mRNA expression of Hsp70 gene in Murrah buffalo in different seasons are represented in Fig. 5. The least squares analysis of variance revealed that the absolute mRNA expression of Hsp70 gene in Murrah buffalo was significantly higher (P ˂ 0.01) in summer season than the spring and winter season prevailing with the calculated THI. The relative expression in terms of fold change values increased by 4.5 times than the spring season whereas found nearly half in winter in comparison to spring.
Fig. 4.
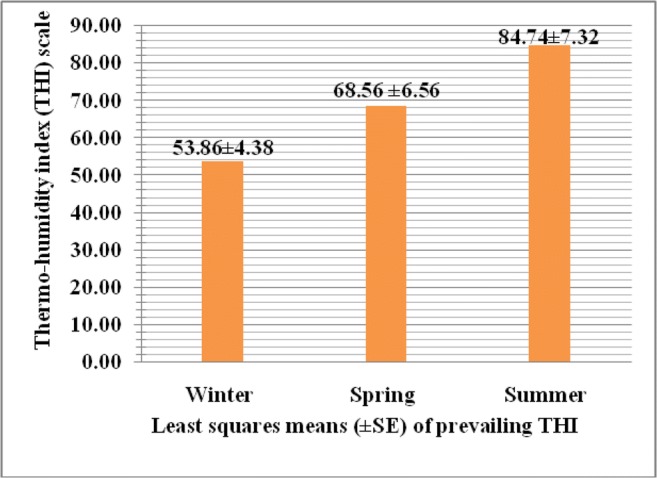
Thermo-humidity index (THI) in different seasons at ICAR-Research Complex for Eastern Region, Patna
Fig. 5.
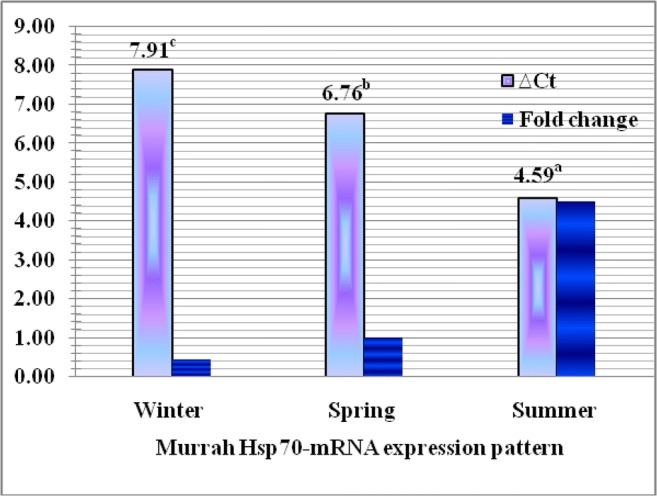
∆Ct values (least squares means) and fold change (geometric means) of mRNA expression of Hsp70 gene in Murrah buffalo in different seasons. Columns with different superscript letters (a,b,c) indicate significant difference (P < 0.01)
Discussion
The PCR-amplified products of Hsp70 gene in Murrah buffalo samples were of 2561 bp size and DNA sequencing by primer walking revealed its four allelic variants of 2511 bp, 2514 bp, 2509 bp, and 2283 bp. Previously, 1926 bp mRNA of Bubalus bubalis Hsp70 indicating 1926-bp coding region (cds) was documented (Pawar et al. 2014a) by sequence analysis and the molecular sizes of the present allelic variants were inclusive of 5′UTR, 3′UTR, and cds. The present allelic sequences had single-nucleotide differences at eight base positions, out of which one substitution was found in the 5′UTR, six substitutions in the coding region and one addition in the 3′UTR. Previously, Indian cattle and buffalo were reported for monomorphic 3′UTR of Hsp70.1 gene while the 5′ flanking region and coding region had 15 single-nucleotide polymorphisms (SNPs) in Buffalo breeds and 19 polymorphic sites in Bos indicus cattle breed (Sodhi et al. 2013). Turkish native cattle demonstrated 13 SNPs and one indel in the Hsp70.1-3′UTR where six SNPs (A101G, C176T, A202G, T209C, A210G, A215G) were transitions and the others were transversions (G63T, T110A, T167A, T174A, T184G, T226A), whereas the 5′UTR had 43 SNPs and three indels including a new C983T nucleotide substitution (Oner et al. 2017). Brahman-influenced cows also demonstrated 11 SNPs in the Hsp70 promoter where eight of the SNPs were transitions (G1013A, G1045A, C1069T, A1096G, G1117A, T1134C, C1154G, T1204C), two transversions (A1125C, G1128T), and one deletion at 895th base position (Banks et al. 2010), indicating that the promoter region was polymorphic. Bos taurus (Angus), Bos indicus (Brahman), and their crossbreds were reported for eight SNPs at nucleotide base position 1851, 1899, 1902, 1917, 1926, 2033, 2087, and 2098 in Hsp70 gene, where only two SNPs resulted in altered peptide sequences known as mis-sense mutations (1926, aspartic acid to glutamic acid, and 2033, glycine to alanine), indicating that most of the SNPs identified might follow breed lineages (Lamb et al. 2007). SNPs in the coding region could affect peptide-binding kinetics or affinity of the Hsp70 proteins and ATPase activity, while nucleotide changes in the flanking regions (promoter and 5′, 3′UTR) might affect inducibility, degree of expression, or stability of Hsp70 mRNA (Lamb et al. 2007). In farm animals, some studies reported possible associations of SNP in the Hsp70 genes with stress response and tolerance to heat. Explicitly, Hsp70.1 and Hsp70.2 are polymorphic, potentially accounting for variation in their functions and susceptibility to stress tolerance (Zhou et al. 2005; Wu et al. 2004; Ross et al. 2003). It is obvious in the present investigation that various alleles were found to have SNPs or mutations, i.e., substitution, addition, and deletion at different positions in the nucleotide sequences. Some of the mutations were silent whereas others expressed themselves by changing the amino acid sequences. If amino acid sequence changes, it results in change in structure and function of polypeptides/proteins. Silent mutations are also important because these mutated sites are more prone to mutation in near future. If mutation occurs as found in the coding region, it may influence the biological activity by changing the amino acid in the polypeptides. If mutation occurs as observed in the non-coding region, it may influence the regulation of gene expression. These changes may affect the different economic traits. If any of these mutations have any significant effect on any economic characters like heat tolerance, production, and reproduction parameter, then it can be identified as genetic marker for particular traits. These genetic markers may be used as a criterion for selection to augment the productivity of the animals. Further, these markers can help in selecting dams and sires for future breeding programs to improve those particular traits in a population.
The highest percent identity of the nucleotide sequences among the allelic variants of Hsp70 in Murrah buffalo indicated this homology towards a highly conserved sequence and the minimum differences that were observed might be a result of different amino acid substitution pattern (Kumar et al. 2015). Furthermore, these sequences also showing the highest percent identity with cattle and Banni buffalo sequences indicated a highly conserved sequence of Hsp70 gene across the breed and species. The evolutionary relationship among the allelic variants of Hsp70 gene where allele B and C formed a cluster indicating their close evolutionary relationship, while allele A was phylogenetically distant from B and C cluster, and D allele was also more distant from A, B, and C cluster. Furthermore, these sequences when compared with the reference sequences in cattle and Banni buffalo showed that the sequences of buffalo allelic variants were in closer cluster than the cattle sequence. Previously, the homology status of Hsp70 was also accessed at the nucleotide and amino acid levels across the species using ClustalW (Version 1.83) indicating that buffalo Hsp70 nucleotide and deduced amino acid sequence was highly conserved across the species (Pawar et al. 2014a). The report (Pawar et al. 2014a) of 98% homology with cattle, goat, and sheep, 96% with camel, 95% with pig, 94% with dog, and 84% with chicken, quail and fish sequences indicates close evolutionary relationship. Previously, 1926-bp-long ORF of Hsp70.1 goat (Capra hircus) when analyzed at nucleotide sequences level, 96–99% similarity appeared at nucleotide level to that of sheep (partial), cattle, and buffalo (Gade et al. 2010). All these reports could corroborate the present findings of percent identity and nucleotide sequence homology share in this investigation.
The Livestock Weather Safety Index (LCI 1970) is commonly used as benchmark to assign heat stress levels to normal, alert, danger, and emergency categories. In this index, thermo-humidity index (THI) ≤ 74 is classified as normal, 75–78 as alert and 79–83 as danger and THI ≥ 84 as emergency, and THI can be used as an indicator of thermal climatic conditions (McDowell et al. 1976). Therefore, based on this index, the present experimental buffaloes reared in summer falls under emergency category indicating stressful condition of the animal. The present investigation envisaged that the absolute mRNA expression of Hsp70 gene in Murrah buffalo was significantly higher (P ˂ 0.01) in summer season than the spring and winter season prevailing with these calculated THI. The present findings along with the relative expression in terms of fold change values increased by 4.5 times than the spring season whereas found nearly half in winter in comparison to spring were in the line of earlier reports as evidenced while significantly higher mRNA expression of Hsp70 in Tarai buffalo was found during summer in comparison to winter with the relative expression values increased by two-fold in summer and decreased by one-fold in winter as compared to thermo-neutral season (Rao et al. 2015). In tropical goats, Hsp70 mRNA expression was reported significantly higher in summer season than the winter indicating heat stress in summer season (Dangi et al. 2012). It was also reported (Mishra et al. 2011) for 200-fold increases in serum Hsp70 mRNA expression levels in Murrah buffalo calves in heat stress condition, and the thermal exposure of Murrah buffalo heifers caused an induction of Hsp70 but declined the lymphocyte proliferative response and interleukin-2, indicating Hsp70 as a marker for heat stress and reduced immune status of buffalo heifers. Differential expression pattern of Hsp70, heat shock transcription factor 1 (HSF1) and cytokines, interleukin-12 (IL-12), tumor necrosis factor- (TNF-), and granulocyte macrophage colony stimulating factor (GMCSF) under heat and cold stress in buffaloes were reported (Pawar et al. 2014b). Specific polymorphisms within Hsp70 gene were found associated with improved heat stress tolerance in Chinese Holstein cattle (Li et al. 2011). The attributable differences in reports for ∆Ct values and fold change of mRNA expression of Hsp70 gene in different seasons might be due to the differences in the ambient temperature associated with prevailing THI where animals were exposed, the different animals and tissue analyzed, and exposed management practices. Earlier study on the expression of Hsp70 mRNA in four different tissues (liver, heart, spleen, and kidney) of Barbari and Jamunapari goat with respect to seasonal variation confirmed that Hsp70 mRNA expression was breed and tissue specific and varied with THI, and this relationship might play an imperative role in conferring thermotolerance against heat stress (Nagayach et al. 2017). The expression pattern of Hsp70 gene was not only breed-specific but also species-specific, most likely due to variations in thermotolerance and adaptation to different climatic conditions (Banerjee et al. 2014). As the heat shock (41 °C) causes increased HSP synthesis, mitochondrial swelling and movement of organelles away from the plasma membrane associated with cytoskeleton reorganization (Rivera et al. 2003; Edwards et al. 1997), the Hsp70 proteins are induced by elevated temperatures or a variety of cellular stresses (Ross et al. 2003) and involved in improved resistance towards stress and disease (Sorensen et al. 2003; Feder and Hofmann 1999; Favatier et al. 1997). Since buffaloes are more prone to heat stress due to the dark body, lesser density of sweat glands (about 1/10 to 1/15 of cattle) (Jayakumar 2014) which together reduces the capacity of cutaneous evaporation, the increase in relative mRNA expression level of Hsp70 gene in Murrah buffalo during summer could suggest their possible role in amelioration of deleterious effect of thermal stress so as to maintain cellular integrity and homeostasis as evidenced by the fact that HSP over expression protects against hyperthermia and cerebral ischemia during the heat stroke (Lee et al. 2006).
Conclusions
It is concluded that Murrah Hsp70 gene is polymorphic with four distinct allelic variants and mutations at different base positions in their nucleotide sequences. The nucleotide and deduced amino acid sequences of the allelic variants were with maximum percent identity, highly conserved within breed and across breed/species, and the minimum divergence were a result of different amino acid substitution pattern. Relative expression level of Murrah Hsp70 mRNA transcripts was 4.5 times more in summer and 0.5 times less in winter than the spring season. It is suggested that a more extensive study based on a large number of observations be taken up in future on genetic profiling of heat shock protein genes along with its proteomics study and contribution of causes thereof on different thermotolrance traits and its implication in terms of further genetic improvement in production performance of Murrah buffalo.
Electronic supplementary material
(DOC 2.28 mb)
Acknowledgments
The authors are thankful to the Director, ICAR-Research Complex for Eastern Region, Patna, Bihar (India) for giving necessary permission towards the first author’s PhD dissertation research work to be carried out at its Division of Livestock and Fishery Management and duly acknowledged the kind cooperation sincerely extended by the Scientists and staff of this Division.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akerfelt M, Trouillet D, Mezger V, Sistonen L. Heat shock factors at a crossroad between stress and development. Ann N Y Acad Sci. 2007;1113:15–27. doi: 10.1196/annals.1391.005. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Mohanarao J, Polley S, Mukherjee A, Das TK, De S. Seasonal variation in expression pattern of genes under Hsp70. Cell Stress Chaperones. 2014;19(3):401–408. doi: 10.1007/s12192-013-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks A, Looper ML, Reiter S, Starkey L, Flores R. Calving traits of crossbred Brahman cows are associated with heat shock protein 70 genetic polymorphisms. Anim Reprod Sci. 2010;119(3):178–182. doi: 10.1016/j.anireprosci.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Basirico L, Morera P, Primi V, Lacetera N, Nardone A, Bernabucci U. Cellular thermotolerance is associated with heat shock protein 70.1 genetic polymorphisms in Holstein lactating cows. Cell Stress Chaperones. 2011;16:441–448. doi: 10.1007/s12192-011-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi SS, Gupta M, Maurya D, Yadav VP, Panda RP, Singh G, Mohan NH, Bhure SK, Das BC, Bag S, Mahapatra R. Expression profile of HSP genes during different seasons in goats (Capra hircus) Trop Anim Health Prod. 2012;44(8):1905–1912. doi: 10.1007/s11250-012-0155-8. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Ealy AD, Monterroso VH, Hansen PJ. Ontogeny of temperature-regulated heat shock protein 70 synthesis in preimplantation bovine embryo. Mol Reprod Dev. 1997;48:25–33. doi: 10.1002/(SICI)1098-2795(199709)48:1<25::AID-MRD4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Favatier F, Bornman L, Hightower LE, Günther E, Polla BS. Variation in HSP gene expression and Hsp polymorphism: do they contribute to differential disease susceptibility and stress tolerance? Cell Stress Chaperones. 1997;2(3):141–155. doi: 10.1379/1466-1268(1997)002<0141:VIHGEA>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI, Sherman MY. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem. 1997;272:18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- Gade Nitin, Mahapatra R. K., Sonawane Arvind, Singh V. K., Doreswamy Ramesh, Saini Mohini. Molecular Characterization of Heat Shock Protein 70-1 Gene of Goat (Capra hircus) Molecular Biology International. 2010;2010:1–7. doi: 10.4061/2010/108429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PJ. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim Reprod Sci. 2004;82-83:349–360. doi: 10.1016/j.anireprosci.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Jayakumar S (2014) Molecular characterization of thermoregulatory genes in Murrah buffaloes. Ph.D. Thesis: ICAR-National Dairy Research Institute, Karnal, Haryana, India
- Kagami H, Nakamura H, Tomita T. Sex identification in chickens by means of the presence of the W chromo-some specific repetitive DNA units. Jap Poult Sci. 1990;27:379–384. doi: 10.2141/jpsa.27.379. [DOI] [Google Scholar]
- Kristensen TN, Lovendahl P, Berg P, Loeschcke V. Hsp72 is present in plasma from Holstein-Friesian dairy cattle and the concentration level is repeatable across days and age classes. Cell Stress Chaperones. 2004;9:143–149. doi: 10.1379/CSC-17.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Gupta ID, Verma A, Verma N, Magotra A, Vineeth MR. Molecular characterization and polymorphism detection in HSPB6 gene in Sahiwal cattle. Indian J Anim Res. 2015;49(5):595–598. [Google Scholar]
- Lacetera N, Bernabucci U, Scalia D, Basirico L, Morera P, Nardone A. Heat stress elicits different responses in peripheral blood mononuclear cells from Brown Swiss and Holstein Cows. J Dairy Sci. 2006;89:4606–4612. doi: 10.3168/jds.S0022-0302(06)72510-3. [DOI] [PubMed] [Google Scholar]
- Lamb M, Okimoto R, Brown M, Rosenkranes C., Jr Associations between cattle breed and heat shock protein 70 gene. AAES Res Series. 2007;545:205–206. [Google Scholar]
- LCI . Patterns of transit losses. Omaha: Livestock Conservation Inc.; 1970. [Google Scholar]
- Lee WC, Wen HC, Chang CP, Chen MY, Lin MT. Heat shock protein 72 overexpression protects against hyperthermia, circulatory shock, and cerebral ischemia during heatstroke. J Appl Physiol. 2006;100(6):2073–2082. doi: 10.1152/japplphysiol.01433.2005. [DOI] [PubMed] [Google Scholar]
- Leppa S, Sistonen L. Heat shock response- pathophysiological implications. Ann Med. 1997;29(1):73–78. doi: 10.3109/07853899708998745. [DOI] [PubMed] [Google Scholar]
- Li Q, Han J, Du F, Ju Z, Huang J, Wang J, Li R, Wang C, Zhong J. Novel SNPs in Hsp70A1A gene and the association of polymorphisms with thermo tolerance traits and tissue specific expression in Chinese Holstein cattle. Mol Biol Rep. 2011;38:2657–2663. doi: 10.1007/s11033-010-0407-5. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the Double Delta CT Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mallick S (2015) Seasonal changes in endocrine profiles, seminal parameters, expression of Hsp genes and influence of prolactin suppression during summer in Karan Fries bulls. MVSc Thesis: ICAR-National Dairy Research Institute, Karnal, India
- McDowell RE, Hooven NW, Adcamoens JK. Effects of climate on performance of Holsteins in first lactation. J Dairy Sci. 1976;59:965–973. doi: 10.3168/jds.S0022-0302(76)84305-6. [DOI] [Google Scholar]
- McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- Mishra A, Hooda OK, Singh G, Meur SK. Influence of induced heat stress on Hsp70 in buffalo lymphocytes. J Anim Physiol Anim Nutr. 2011;95(4):540–544. doi: 10.1111/j.1439-0396.2010.01082.x. [DOI] [PubMed] [Google Scholar]
- Nagayach R, Gupta UD, Prakash A. Expression profiling of hsp70 gene during different seasons in goats (Capra hircus) under sub-tropical humid climatic conditions. Small Rumin Res. 2017;147:41–47. doi: 10.1016/j.smallrumres.2016.11.016. [DOI] [Google Scholar]
- National Research Council . A guide to environmental research on animals. Washington: Nat Acad Sci; 1971. [Google Scholar]
- Oner Y, Keskin A, Ustuner H, Soysal D, Karakaş V. Genetic diversity of the 3′ and 5′ untranslated regions of the HSP70.1 gene betweennative Turkish and Holstein Friesian cattle breeds. S Afr J Anim Sci. 2017;47(4):424–439. doi: 10.4314/sajas.v47i4.2. [DOI] [Google Scholar]
- Pawar HN, Agrawal RK, Ramneek BGS. In silico analysis of stress inducible 70 kDa heat shock protein of Bubalus bubalis. Int J Adv Res. 2014;2(2):1480–1494. [Google Scholar]
- Pawar HN, Kumar GVPPSR, Narang R, Agrawal RK. Heat and cold stress enhances the expression of heat shock protein70, heat shock transcription factor 1 and cytokines (IL-12, TNF- and GMCSF) in buffaloes. Int J Cur Microbiol Appl Sci. 2014;3(21):307–317. [Google Scholar]
- Rao M, Yadav M, Ramesh K, Uniyal S, Rastogi SK, Sejian V, Hyder I. Hsp70 as a marker of heat and humidity stress in Tarai buffalo. Trop Anim Health Prod. 2015;47(1):111–116. doi: 10.1007/s11250-014-0692-4. [DOI] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40(2):253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Rivera RJ, Kelley KL, Erdos GW, Hansen PJ. Alterations in ultrastructural morphology of two-cell bovine embryos produced in vitro and in vivo following a physiologically relevant heat shock. Biol Reprod. 2003;69:2068–2077. doi: 10.1095/biolreprod.103.020347. [DOI] [PubMed] [Google Scholar]
- Ross OA, Curran MD, Crum KA, Rea IM, Barnett YA, Middleton D. Increased frequency of the 2437 T allele of the heat shock protein 70-hom gene in an aged Irish population. Exp Gerontol. 2003;38:561–565. doi: 10.1016/S0531-5565(03)00006-8. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- SAS (2008) SAS/STAT 9.2 User’s Guide. Cary:SAS Institute Inc.
- Sodhi M, Mukesh M, Kishore A, Mishra BP, Kataria RS, Joshi BK. Novel polymorphisms in UTR and coding region of inducible heat shock protein 70.1 gene in tropically adapted Indian zebu cattle (Bos indicus) and riverine buffalo (Bubalus bubalis) Gene. 2013;527(2):606–615. doi: 10.1016/j.gene.2013.05.078. [DOI] [PubMed] [Google Scholar]
- Sorensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Literat. 2003;6:1025–1037. [Google Scholar]
- Thiruvenkadan AK, Rajendran R, Muralidharan J. Buffalo genetic resources of India and their conservation. Buffalo Bull. 2013;32:227–235. [Google Scholar]
- Tukey JW (1953) The problem of multiple comparisons. Unpublished manuscript. In: The Collected Works of John W Tukey VIII. Multiple Comparisons: 1948-1983; Chapman and Hall, New York, pp. 1-300
- Wimmers K, Ponsuksili S, Hardge T, Valle-Zarate A, Mathur PK, Horst P. Genetic distinctness of African, Asian and South American local chickens. Anim Genet. 2000;31:159–165. doi: 10.1046/j.1365-2052.2000.00605.x. [DOI] [PubMed] [Google Scholar]
- Wu YR, Wang CK, Chen CM, Hsu Y, Lin SJ, Lin YY, Fung HC, Chang KH, Lee-Chen GJ. Analysis of heat-shock protein 70 gene polymorphisms and the risk of Parkinson’s disease. Hum Genet. 2004;114:236–241. doi: 10.1007/s00439-003-1050-1. [DOI] [PubMed] [Google Scholar]
- Zhou F, Wang F, Li F, Yuan J, Zeng H, Wei Q, Tanguay RM, Wu T. Association of hsp70.2 and hsp-hom gene polymorphisms with risk of acute high-altitude illness in a Chinese population. Cell Stress Chaperones. 2005;10:349–356. doi: 10.1379/CSC-156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 2.28 mb)