Abstract
The use of induction immunosuppression in liver transplantation (LT) remains controversial. This was a retrospective cohort study of adult, first-time liver alone recipients (N=69,349) at 114 U.S. centers between 2005–2018 using data from the United Network for Organ Sharing. The comparative effectiveness of non-depleting and depleting induction (NDI and DI) was assessed. Overall, 27% of recipients received induction with 65.7% of the variance in the receipt of induction being attributed to transplant center alone. NDI and DI were associated with a lower risk of death/graft failure compared to no induction (adjusted hazard ratio 0.90 (95% CI: 0.86–0.95) and 0.91 (95% CI: 0.85–0.97), respectively; p<0.001). In non-dialysis recipients at the mean transplant estimated glomerular filtration rate (eGFR), NDI was associated with an adjusted gain in eGFR by 6 months of +3.8mL/min/1.73m2 and DI of +3.33mL/min/1.73m2 compared to no induction (p<0.001). Recipients with lower eGFR at LT had greater predicted improvement in eGFR (interaction p<0.001). Only NDI was associated with a reduced risk of acute rejection in the first year post-LT (odds ratio 0.87, 95% CI: 0.8–0.94). Significant variability in induction practices exists with center being a major determinant. The absolute incremental benefits of NDI and DI over no induction were small.
Introduction
Induction therapy is potent immunosuppression (IS) given immediately prior to solid organ transplantation. While it is commonly employed in kidney, heart and lung transplantation, its use in liver transplantation (LT) remains controversial(1–13). The proposed benefits of induction IS include improved post-transplant renal function due to calcineurin inhibitor (CNI) minimization, a reduced risk of acute rejection and decreased corticosteroid dependence(14, 15). However, the evidence supporting induction in LT is of limited quality due to study size and heterogeneity(16, 17). There is currently no consensus guidance on the use of induction therapy for LT by the American Association for the Study of Liver Diseases(18). Nevertheless, its use has increased significantly over time, a shift that has taken place despite a lack of drug approval for this indication(19–23).
In the era of ‘big data’, observational research using real-world evidence to assess the effectiveness of therapies in day-to-day practice has gained momentum. This approach can complement the findings obtained under ideal circumstances from smaller clinical trials, which may lack generalizability and reproducibility(24, 25). As part of the 21st Century Cures Act, the U.S. Food and Drug Administration (FDA) increasingly relies on real-world evidence for drug regulation and post-marketing evaluation(26, 27). To date, population-level comparative effectiveness research of pharmacologic therapies in solid organ transplantation remains limited. This, in combination with the significant heterogeneity of published clinical trials in transplant medicine, has contributed to substantial practice variability across centers.
Center differences in maintenance IS strategies beyond that which is expected due to recipient factors has been demonstrated(28, 29). However, practice patterns related to the recent increased use of induction therapy have not been investigated on a national level. Moreover, the comparative effectiveness of induction therapies in LT has not been studied in a manner that accounts for recent changes in recipient characteristics, including the increase in the number of older recipients and the concurrent decline of those with active Hepatitis C virus (HCV)(23). Uemura et al previously found significantly worse survival in patients with HCV who received anti-thymocyte globulin (ATG) using United Network from Organ Sharing (UNOS) data from 2002–2007(30). It is unknown whether this concern persists in the era of highly-effective direct-acting antivirals that allow for prompt treatment of HCV post-LT.
The objectives of this study were two-fold: 1) to evaluate center practice variability in the use and type of induction IS across the U.S., 2) to assess the comparative effectiveness of lymphocyte depleting and non-depleting induction in a contemporary, national sample of LT recipients with regards to mortality or graft failure, post-LT renal function and acute rejection.
Methods
Data Source & Study Population
This was a retrospective cohort study using data from UNOS. All adult (≥18 years) LT recipients between 1/1/2005–1/31/2018 were potentially eligible for inclusion. Subjects were excluded if they had previously undergone LT or received multiple organs at the time of LT (e.g. liver-kidney, liver-lung, liver-heart), as IS decision-making in such recipients is inherently different. Subjects at centers performing <50 LTs during the study period (i.e. less than 5 LTs per year) were also excluded. These centers were primarily pediatric centers transplanting a small number of young adults. Lastly, due to their small number, patients receiving induction agents other than basiliximab, daclizumab, thymoglobulin, alemtuzumab and rituximab were also excluded.
Variables
Recipient clinical factors evaluated in analyses included: age, gender, liver disease etiology, race/ethnicity, history of hepatocellular carcinoma, Karnofsky Performance Score, location immediately prior to LT (home, inpatient ward, intensive care unit), need for pre-LT hemodialysis, and whether the patient was Status 1 at LT (i.e., emergent LT). Recipient laboratory values at the time of LT included serum total bilirubin, albumin, international normalized ratio (INR), and estimated glomerular filtration rate (eGFR) by Modification of Diet in Renal Disease 4 equation(31). Donor factors included donor age and whether the organ was procured from a donor after circulatory determination of death (DCDD). Temporal trends by transplant year and geographic influences by transplant center were also assessed.
Receipt of induction IS was evaluated both as a binary variable (no induction, induction) and as a three-level categorical variable (no induction, non-depleting induction [NDI] and depleting induction [DI]). Subjects with no reported induction regimen were categorized as not having received induction. NDI included the interleukin-2 receptor antagonists daclizumab and basiliximab. DI included thymoglobulin, alemtuzumab and rituximab. The association of induction use/type and early maintenance regimen selection was assessed descriptively. However, early maintenance IS was not included in multivariable analyses as the objective of this research was to simulate actual decision-making at the time of LT and because early maintenance immunosuppression is believed to be in the causal pathway linking induction IS to post-LT outcomes(32). Primary post-transplant outcomes evaluated included: 1) composite end-point of all-cause mortality and graft failure (time-to-event: alive with initial graft vs died or retransplanted); 2) change in eGFR between LT and 6 months post-LT (continuous, in mL/min/1.73m2); 3) biopsy-proven acute rejection (BPAR) in the first year post-LT, as reported to UNOS (binary: no rejection vs rejection)(33).
Analytical Methods
Recipient factors associated with induction use were assessed using Chi-squared tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Unadjusted center variability in induction practices across the US were described. The proportion of variation in the receipt of induction attributed to transplant center was obtained by fitting a mixed-effects multivariable logistic regression using receipt of induction as a binary outcome with recipient and donor factors specified as fixed effects, and transplant center as a random effect. From this model, the intraclass correlation coefficient (ICC,) was obtained using the following equation: , where is the variance of the random intercept. In this context, the ICC is the proportion of total variability in induction use that is explained by variance between transplant centers independent of specified recipient and donor factors(34).
Survival analyses evaluated the association of induction use/type as a three-level categorical variable and the composite end-point of retransplantation or death. Patients were censored at date of last follow-up or at the end-date of the study period (i.e., 1/31/2018). The unadjusted probability of survival with the initial LT graft was assessed using Kaplan Meier curves and the log-rank test. Multivariable Cox proportional hazards models were fit adjusted for recipient and donor factors with center specified as a shared frailty term to account for correlation within centers. The proportional hazards assumption was confirmed graphically with scaled Schoenfeld residual and log-log plots. From these models, adjusted hazard ratios (aHRs) of NDI and DI were obtained using no induction as the reference, and the adjusted probability of survival with the initial LT graft according to induction use/type was evaluated via marginal standardization.
The effect of induction use/type on early post-LT renal function was assessed in dialysis and non-dialysis LT recipients using multivariable mixed-effects linear regression with change in eGFR from LT to 6 months post-LT as a continuous outcome and center specified as a random effect. Variation in the effect of induction according to baseline eGFR in non-dialysis recipients was also investigated, as it was hypothesized that the degree of benefit of induction could vary depending on baseline renal function. A secondary analysis evaluated the association of induction use/type on change in eGFR from LT to 1 year post-LT in dialysis and non-dialysis recipients. Lastly, multivariable mixed-effects logistic regression models with center specified as a random effect to account for clustering was used to compute odds ratios (ORs) for the association of BPAR in the first year after LT with induction regimen, contingent on surviving the first year post-LT. Factors known to be associated with the risk of acute rejection, such as age, gender, etiology of liver disease and race/ethnicity were evaluated as interaction terms with induction use/type. From both final multivariable mixed-effects models, marginally standardized predictions of the change in eGFR after LT and of the probability of BPAR after LT were obtained.
All multivariable models were adjusted for all components listed in the section Variables of the Methods section, unless otherwise stated. Complete case analysis was used for all analyses.
Results
The final study cohort included 69,349 LT recipients at 114 transplant centers across the US. Overall, 18,755 (27%) received induction at LT, of which 60.8% received NDI and 39.2% received DI.
Recipient and donor factors associated with induction
Basic demographic and clinical factors by induction use/type are shown in Table 1. There was little variation in liver disease etiology by induction practice. For example, among those receiving no induction, 42.9% had Hepatitis B or C, whereas these patients accounted for 38.4% and 39.5% of those receiving NDI and DI, respectively. Similarly, 11.7% of patients not receiving induction had auto-immune liver disease (included auto-immune hepatitis, primary sclerosing cholangitis and primary biliary cirrhosis), compared to 11.2% of those receiving NDI and 13.2% of those receiving DI. Renal dysfunction at LT was associated with the use of NDI more so than DI. NDI was used in 23.6% of those with eGFR <30mL/min/1.73m2 versus 13.3% of those with eGFR ≥60mL/min/1.73m2. In contrast, DI was used in 10.4% of those with eGFR <30mL/min/1.73m2 versus 10.4% of those with eGFR ≥60mL/min/1.73m2. The frequency of induction type used was similar among recipients of DCDD organs, with 14.1% receiving NDI and 14.7% receiving DI.
Table 1:
Recipient demographic and clinical characteristics by induction use/type (N=69,349)
| Variable | No induction (N=50,594) | Non-depleting (N=11,401) | Depleting (N=7,354) | P-value |
|---|---|---|---|---|
| Male, N (%) | 34,005 (67.21) | 7,491 (65.7) | 4,913 (66.81) | 0.008 |
| Age, median (IQR) | 56 (50–62) | 56 (50–62) | 56 (49–62) | 0.02 |
| Race/Ethnicity, N (%) | <0.001 | |||
| White | 36,315 (71.78) | 8,175 (71.7) | 5,488 (74.63) | |
| Black | 4,499 (8.87) | 1,024 (8.98) | 667 (9.07) | |
| Hispanic | 6,813 (13.47) | 1,545 (13.55) | 835 (11.35) | |
| Asian | 2,351 (4.65) | 451 (3.96) | 299 (4.07) | |
| Other | 627 (1.24) | 206 (1.81) | 65 (0.88) | |
| Liver disease, N (%) | <0.001 | |||
| HCV/HBV | 21,695 (42.88) | 4,383 (38.44) | 2,901 (39.45) | |
| Alcohol | 8,638 (17.07) | 2,106 (18.47) | 1,357 (18.45) | |
| Fatty liver | 8,056 (15.92) | 2,332 (20.45) | 1,345 (18.29) | |
| Auto-immune* | 5,913 (11.69) | 1,275 (11.18) | 973 (13.23) | |
| Other | 6,292 (12.44) | 1,305 (11.45) | 778 (10.58) | |
| Lab MELD, median (IQR) | 19 (13–28) | 23 (15–33) | 19 (13–28) | <0.001 |
| Dialysis at LT, N (%) | 3,888 (7.69) | 1,342 (11.8) | 550 (7.49) | <0.001 |
| HCC within Milan, N (%) | 13,139 (25.97) | 2,294 (20.12) | 1,505 (20.47) | <0.001 |
Includes auto-immune hepatitis, primary sclerosing cholangitis and primary biliary cirrhosis
Abbreviations: IQR – inter-quartile range; HCV – Hepatitis C virus; HBV – Hepatitis B virus; MELD – Model for End-stage Liver Disease score; LT – liver transplantation; HCC – hepatocellular carcinoma
Early maintenance immunosuppression
Patients receiving no induction were most often discharged from the hospital on triple therapy (65.7%) with a calcineurin inhibitor (i.e. tacrolimus, cyclosporine), anti-metabolite (i.e., azathioprine, mycophenolic acid) and corticosteroids, or dual therapy with CNI and corticosteroids (13.7%). Use of NDI was also associated with a predominance of triple therapy at discharge (60.8%), but with dual therapy with CNI and anti-metabolite being the second most common (24%). Among recipients receiving DI, early maintenance IS choice was different and more heterogeneous with 43.6% receiving dual therapy with CNI and anti-metabolite, 27.9% triple therapy and 18.1% CNI monotherapy at hospital discharge.
Temporal and geographic factors associated with induction
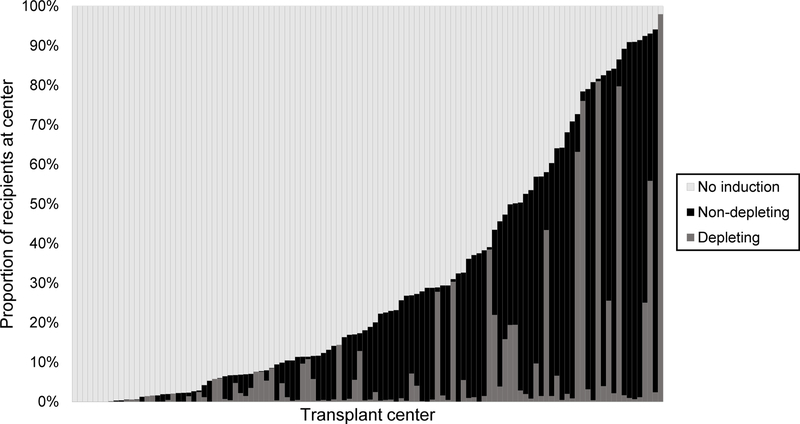
There was minimal change in the use of DI over time, which increased from 9.2% of LTs in 2005–2006 to 11.4% in 2016–2017, whereas the proportion of LTs receiving NDI nearly doubled (10.5% in 2005–2006 to 19.6% in 2016–2017). There was significant among-center variability in the use of induction overall and by induction type (Figure 1). Among the 114 centers, 85 (74.6%) used no induction in over 50% of LTs. Of the 29 centers using induction in a majority of LTs, 19 used NDI in over half of recipients and 6 predominantly used DI. The ICC, or proportion of variance in the receipt of induction attributed to transplant center independent of recipient and donor factors, was found to be 65.7%.
Figure 1.
Center variability in the use and type of induction at liver transplantation (N=114 centers)
Note: Each vertical bar represents one transplant center
Post-transplant survival and graft failure
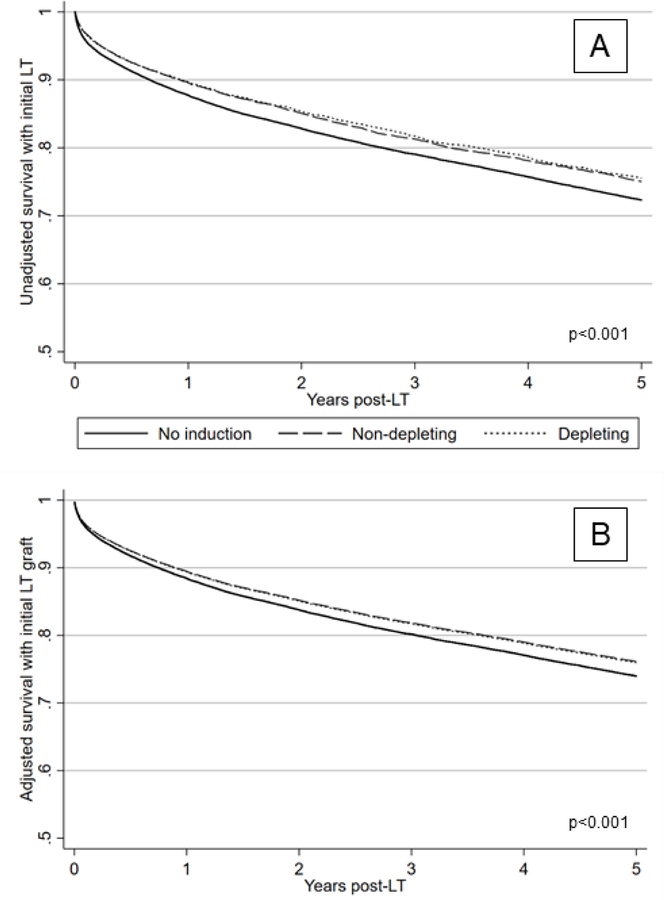
During follow-up, 4.4% (2,936/66,890) of observed subjects were retransplanted (i.e., graft failure) and 24.1% died (16,172/67,202). The median follow-up was 3.1 years (interquartile range [IQR]: 1.0–6.9 years). The distribution of time to retransplant and/or death was significantly different by induction use/type (p<0.001; Figure 2A). However, the absolute difference in the unadjusted survival probability was small: for example, at 3-years for no induction this was 0.79 (95% CI: 0.79–0.79), for NDI 0.81 (95% CI: 0.80–0.82) and for DI 0.82 (95% CI: 0.81–83). Receipt of NDI and DI was associated with a significantly lower adjusted hazard of retransplant and/or death compared to not receiving induction (aHR 0.90, 95% CI: 0.86–95, for NDI and 0.91, 95% CI: 0.85–0.97, for DI; composite hypothesis test p<0.001). Similar to the unadjusted analysis, the absolute difference in the adjusted probability of survival without retransplantation by induction use/type was small (Figure 2B).
Figure 2.
Unadjusted and adjusted probability of survival without retransplantation by induction use and type
Panel A: N=68,752
Panel B: N=68,657
Patient/graft survival in Panel B was adjusted for the following covariates: age, gender, liver disease etiology, race/ethnicity, history of hepatocellular carcinoma, Karnofsky Performance Score, location prior to LT, hemodialysis, Status 1 at LT, bilirubin at LT, albumin at LT, INR at LT, eGFR at LT, donor age, DCDD organ, transplant year and transplant center.
Abbreviations: DCDD – donation after circulatory determination of death; eGFR – estimated glomerular filtration rate; INR – international normalized ratio; LT – liver transplantation
Post-transplant renal function
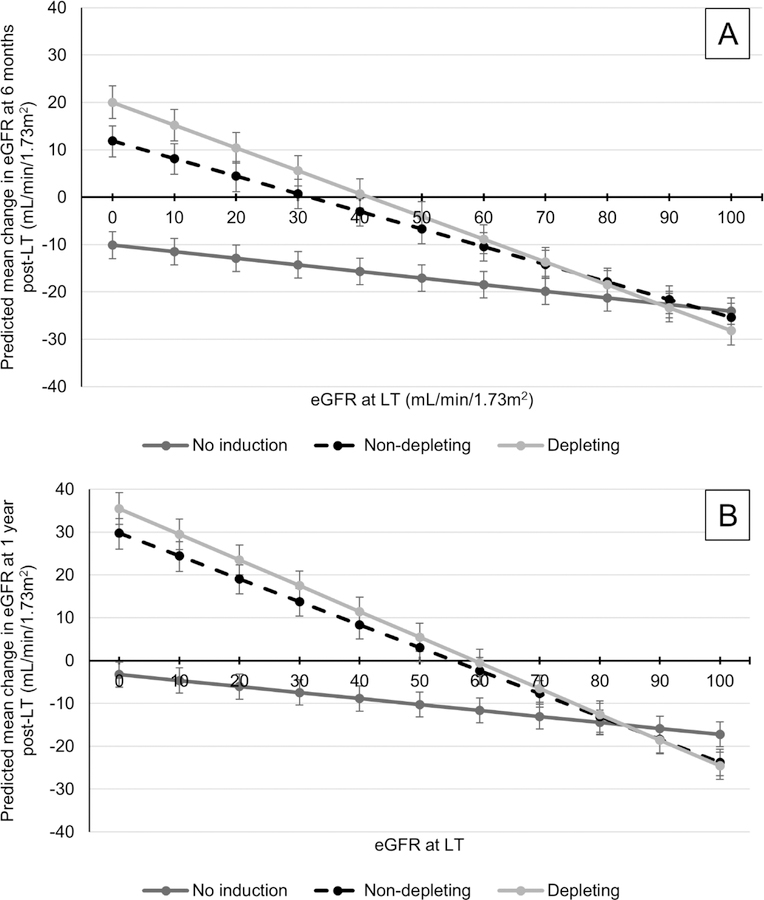
Data on renal function at 6 months post-LT were available from 45,282 recipients (65.3%). In recipients not on dialysis at LT, eGFR decreased by a median of 11.33mL/min/1.73m2 (IQR: −32.98 to +10.11mL/min/1.73m2) at 6 months post-LT. In the unadjusted analysis, NDI improved eGFR at 6 months post-LT by +9.90mL/min/1.73m2 (95% CI: 8.80–11.00) and DI by +7.18 mL/min/1.73m2 (95% CI: 5.67–8.68; no induction as reference; composite hypothesis test p<0.001). The interaction of eGFR at LT and induction use/type was statistically significant (p<0.001). In non-dialysis recipients at the mean eGFR at LT (i.e., 78.26mL/min/1.73m2), NDI was associated with an adjusted gain of +3.80mL/min/1.73m2 (95% CI: 2.86–4.74mL/min/1.73m2) at 6 months post-LT compared to no induction, and DI a gain of +3.33mL/min/1.73m2 (95% CI: 2.06–4.60mL/min/1.73m2; composite hypothesis test p<0.001).
As reference, unadjusted predicted mean change in eGFR at 6 months according to induction use/type and baseline eGFR among non-dialysis recipients is reported in Supplemental Figure 1. Panel A of Figure 3 demonstrates the adjusted predicted mean change in eGFR at 6 months post-LT by induction use/type and baseline eGFR in non-dialysis recipients. All recipients not receiving induction were predicted to have a decrease in eGFR between LT and 6 months post-LT. Compared to no induction, NDI and DI were associated with less of a reduction in eGFR at 6 months post-LT with eGFR at LT <70mL/min/1.73m2. DI was associated with a gain in eGFR at 6 months when eGFR <40 mL/min/1.73m2 at LT, and NDI when eGFR <30 mL/min/1.73m2 at LT.
Figure 3.
Predicted mean change in eGFR between LT and 6 months (Panel A) and 1 year (Panel B) post-LT in recipients not on dialysis at LT
Panel A: N=41,622
Panel B: N=42,069
Results adjusted for the following covariates: age, gender, liver disease etiology, race/ethnicity, history of hepatocellular carcinoma, Karnofsky Performance Score, location prior to LT, Status 1 at LT, bilirubin at LT, albumin at LT, INR at LT, donor age, DCDD organ, transplant year and transplant center.
Vertical bars represent 95% confidence intervals for each point estimate.
Abbreviations: DCDD – donation after circulatory determination of death; eGFR – estimated glomerular filtration rate; INR – international normalized ratio; LT – liver transplantation
As a secondary analysis, the association of induction use/type and change in eGFR at 1 year post-LT was evaluated among 45,527 patients with available data (65.6%). NDI was associated with an adjusted gain of +2.09mL/min/1.73m2 (95% CI: 1.11–3.08) and DI a gain of +2.68 mL/min/1.73m2 (95% CI: 1.33–4.02) between LT and 1 year post-LT among non-dialysis recipients at the mean eGFR at LT (no induction as reference; composite hypothesis test p<0.001). Panel B of Figure 3 demonstrates the adjusted predicted mean change in eGFR between LT and 1 year post-LT according to induction use/type and eGFR at LT. Significantly greater improvements in eGFR at 1 year were predicted among induction recipients with eGFR <60mL/min/1.73m2 and not on dialysis at LT.
Overall, 8.4% of the cohort received dialysis prior to LT. In these recipients, eGFR at 6 months improved by a median of +17.69 mL/min/1.732 (IQR: −4.98 to +37.19mL/min/1.73m2). Post-LT dialysis status was not available from UNOS data. In recipients on dialysis prior to LT, induction use/type was not a significant predictor of change in eGFR between LT and 6 months post-LT in both unadjusted and adjusted analyses (composite hypothesis test p=0.07 in both models). Between LT and 1 year post-LT, DI was associated with a significant gain of +6.65mL/min/1.73m2 (95% CI: 1.53–11.78) in dialysis recipients, whereas NDI was not significantly different from no induction (β=−0.65, 95% CI: −4.06 to +2.76; composite hypothesis test p=0.03).
Rejection in the first post-LT year
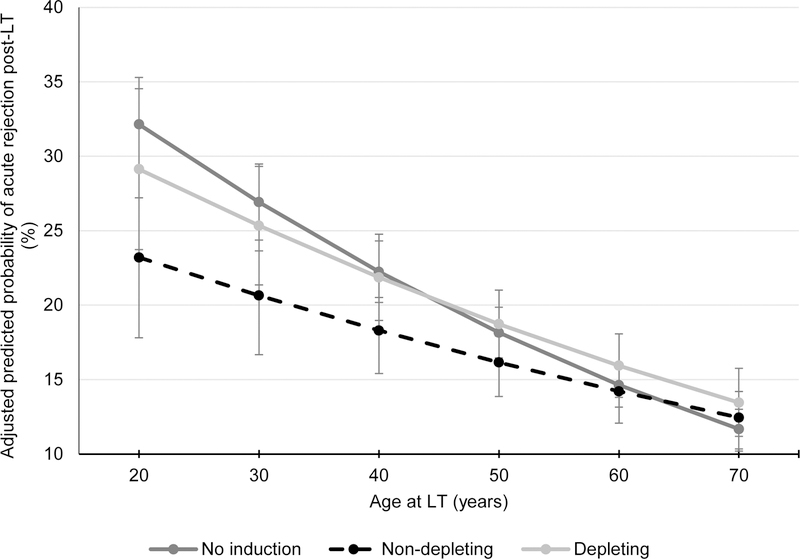
Among 44,276 recipients alive 1 year post-LT with available follow-up data (63.8% of the cohort), 16.3% experienced at least one episode of BPAR in the first year post-LT. The proportion of recipients with BPAR was 16.5% with no induction, 16.2% with NDI and 14.9% with DI (p=0.014). NDI was associated with a 13% reduction in the unadjusted odds of BPAR compared to no induction (OR 0.87, 95% CI: 0.8–0.94). In contrast, the OR with DI was not statistically significant (OR 1.11 versus no induction, 95% CI: 0.99–1.24; composite hypothesis test p<0.001).
The interaction of induction use/type with age was statistically significant (p=0.002). However, the interactions with gender, race/ethnicity and etiology of liver disease were not (p= 0.7, 0.5 and 0.1, respectively). In adjusted analyses, NDI was associated with a 12% reduction in the odds of BPAR in the first year compared to no induction (OR 0.88, 95% CI: 0.81–0.96) for average-aged recipients. The adjusted OR of BPAR using DI was not significantly different from that with no induction in the multivariable model (OR 1.07, 95% CI: 0.95–1.20; composite hypothesis test p=0.004). Figure 4 demonstrates the reduction in adjusted predicted probability obtained through marginal standardization of BPAR in the first year post-LT with decreasing age at LT in recipients receiving NDI.
Figure 4.
Adjusted predicted probability of BPAR in the first year according to age and induction use/type among recipients surviving 1 year post-LT (N=44,207)
Results adjusted for the following covariates: gender, liver disease etiology, race/ethnicity, history of hepatocellular carcinoma, Karnofsky Performance Score, location prior to LT, hemodialysis, Status 1 at LT, bilirubin at LT, albumin at LT, INR at LT, eGFR at LT, donor age DCDD organ, transplant year and transplant center.
Vertical bars represent 95% confidence intervals for each point estimate.
Abbreviations: BPAR – biopsy-proven acute rejection; DCDD – donation after circulatory determination of death; eGFR – estimated glomerular filtration rate; INR – international normalized ratio; LT – liver transplantation
Table 2 summarizes the key findings of this study.
Table 2:
Summary table of the association of induction with post-transplant outcomes adjusted for recipient and donor factors
| ReLT or death, aHR (95% CI) | eGFR change at 6 months post-LT*, mL/min/1.73m2 (95% CI) | eGFR change at 1 year post-LT*, mL/min/1.73m2 (95% CI) | BPAR at 1 year post-LT†, aOR (95% CI) | |
|---|---|---|---|---|
| No induction | Reference | Reference | Reference | Reference |
| NDI | 0.90 (0.86–0.95) | +3.80 (2.86–4.74) | +2.09 (1.11–3.08) | 0.88 (0.81–0.96) |
| DI | 0.91 (0.85–0.97) | +3.33 (2.06–4.60) | +2.68 (1.33–4.02) | 1.07 (0.95–1.20) |
In recipients not on dialysis at LT; given significant interaction with baseline eGFR, point estimate reported is at the mean eGFR at LT of 78.26mL/min/1.73m2
† Given significant interaction with age, point estimated reported is at the mean age at LT of 55 years
Abbreviations: aHR – adjusted hazard ratio; aOR – adjusted odds ratio; BPAR – biopsy-proven acute rejection; CI – confidence interval; DI – depleting induction; eGFR – estimated glomerular function; LT – liver transplantation; NDI – non-depleting induction; reLT - retransplantation
Discussion
In all aspects of medicine, treatment selection is influenced by patient factors and physician preferences. The decision to employ induction IS at the time of LT is no different: transplant physicians must weigh the anticipated risks and benefits of induction therapy for each recipient in the context of their center’s abilities and experience. But, as a transplant community, we must also strive to reduce any unnecessary variability that may compromise outcomes, giving each an equal opportunity to thrive after LT. This study quantifies the significant heterogeneity in induction practices across the U.S. and demonstrates that recipients are far more likely to receive induction on the basis of where they are transplanted, as opposed to intrinsic characteristics predictive of greater anticipated benefit.
There have been no randomized controlled trials comparing NDI to DI in over two decades(16). Nevertheless, transplant centers across the world are increasingly adopting an early IS strategy that includes NDI. Guidelines from the European Association for the Study of the Liver recommend NDI in recipients with renal dysfunction at LT, and the recent guidelines from the Asian Liver Transplant Network advise the administration of NDI in all LT recipients irrespective of pre-LT renal function(35, 36). In the U.S., the number of LTs performed with NDI over the last decade has nearly doubled with 21% of U.S. LT centers using NDI in ≥50% of LTs in 2017. This shift in IS management worldwide has occurred despite a lack of strong data supporting the use of induction in LT.
This comparative effectiveness study demonstrated a small statistically significant improvement in patient/graft survival in those who received induction IS, although the absolute difference in survival was small. Renal outcomes at 6-months and 1-year post-LT were statistically better but clinically small among those receiving NDI and DI. These differences were more pronounced in patients with more impaired renal function but not on dialysis at LT – an important finding as many prior trials excluded patients with advanced renal dysfunction at LT(16, 17, 37–40). Lastly, NDI, but not DI, reduced the risk of BPAR in the first post-LT year, with a more pronounced effect in younger recipients. When considering the cohort characteristics overall, the incremental benefits of induction were small for most LT recipients.
The substantial costs associated with these therapies must be considered. In the U.S., rabbit ATG (Thymoglobulin, Genzyme) and basiliximab (Simulect, Novartis) are the sole DI and NDI induction therapies currently approved by the FDA for rejection prophylaxis in kidney transplant (KT) recipients. These drugs are typically dosed once immediately prior to KT, with follow-up dosing daily for 4–7 days post-operatively for rabbit ATG, and on post-operative day 4 for basiliximab. Thus, U.S. average wholesale price for rabbit ATG ranges approximately $13,193–23,087 per KT recipient (using standard 1.5mg/kg dosing in a 60kg patient), and is approximately $8,946 per KT recipient for basiliximab(41). This research was not able to evaluate potential risks associated with induction therapy, such as post-LT cytomegalovirus infections or post-transplant lymphoproliferative disorder. However, these issues must be factored into any cost-effectiveness analysis of induction IS in LT, particularly given their typically low prevalence in LT(42, 43).
In KT recipients, recently published economic evaluations of NDI and DI have been favorable, with ATG being often preferred(3, 44, 45). However, cost-effectiveness studies of induction in LT remain extremely limited(46–48). Dopazo et al found comparable improvements in post-LT renal function and rejection risk, but significant cost-savings at their hospital in Spain, when using a low-dose equine ATG (ATGAM; Pfizer) protocol versus standard basiliximab in recipients with pre-LT renal impairment(48). Further studies are needed to estimate the financial impact of a more uniform use of induction in adult LT recipients in the U.S. In the current era of value-based medicine, a personalized, rather than a ‘one size fits all’, approach to clinical decision-making is also critical to cost containment. As demonstrated in our study, it is likely that the benefits of induction are greatest in certain recipients. Thus, any future cost-effectiveness research in this area must also identify which LT recipient subpopulations benefit most from induction IS.
IS regimen at LT discharge differed significantly by induction IS strategy. Early maintenance IS is undoubtedly in the causal pathway linking induction therapy to post-LT outcomes, and as a result was not accounted for in the analyses presented(32). A key example of this is the observed improvement in post-LT renal function with induction therapy, which itself has no known direct nephroprotective effects. The lack of data regarding maintenance IS drug dosing or serologic levels in the UNOS database prevented further evaluation of the benefits of induction therapy independent of those related to early maintenance IS. For instance, most NDI recipients were discharged on a 3-drug maintenance regimen (CNI, anti-metabolite and steroids), similar to patients not receiving induction, and whether the observed decrease in BPAR with NDI occurred in the setting of standard or dose-reduced maintenance IS could not be determined.
Missing data is an inherent limitation of retrospective observational studies. In this study, 25% of recipients surviving at least 6 months post-LT had missing renal function data, and 19% of those surviving at least 1 year were missing rejection data. These outcomes are collected by UNOS from post-LT office visits, such that missing data may have resulted from non-compliance, or subjects being in a poor or good state of health. It is therefore possible that some data were missing not completely at random. While mixed-effects regression models are robust to random missingness, they are subject to bias when data are missing not at random in which unobserved subjects are more (or less) likely to experience the outcome of interest than patient with complete data(49, 50).
It has also been shown that approximately 15% of BPAR episodes are missed in UNOS data, though the positive predictive value of UNOS reporting is high(33). Moreover, it could not be determined whether induction IS reduced the severity of rejection episodes as rejection grade is not available from UNOS data. Due to significant missing data, changes in post-LT renal function beyond 1 year were not explored, though prior research indicates that little change occurs after the first year post-LT(51, 52). In addition, dialysis status after LT was not available, limiting the interpretation of changes in eGFR post-LT. Details related to induction protocols were also not available. Lastly, other important outcomes potentially influenced by induction IS could not be explored, such as post-LT diabetes.
Given its marked increase in use over the last decade, there is a great need for cost-effectiveness research, as well as consensus guidance, on the use of induction IS for LT in the U.S. Marked center variability in induction practices was demonstrated, such that where one undergoes LT was the primary determinant of receiving induction. In this population-level study, both induction types had minimal overall effects on patient/graft survival, post-LT renal function and BPAR. However, the benefits of NDI and DI were not uniform across all recipients. Addressing these center-based practice differences and adopting a more consistent approach to induction in LT may represent an opportunity for improved outcomes overall.
Supplementary Material
Acknowledgments
This work was supported by a Career Development Award from the National Institutes of Health (Principal Investigator: Therese Bittermann; 1-K08-DK117013–01). The authors wish to acknowledge the Center for Pharmacoepidemiology Research and Training at the University of Pennsylvania for providing access to the Cerner Multum drug database.
Therese Bittermann receives research grant funding from the National Institutes of Health. Rebecca Hubbard receives research grant funding from the National Institutes of Health and from Humana. James Lewis receives consulting honoraria from Johnson & Johnson Consumer Inc., Takeda, Merck, Celgene, Janssen Pharmaceuticals, AbbVie, Eli Lilly and Company, Samsung Bioepis, Dark Canyon Laboratories, Bridge Biotherapeutics Inc., Bristol-Myers Squibb. He also serves on the Data Safety Monitoring Board for Pfizer, Gilead and UCB, and receives research grant funding from Takeda and Janssen. David Goldberg receives research grant funding from Merck, Zydus and Gilead.
Abbreviations
- aHR
adjusted hazard ratio
- ATG
anti-thymocyte globulin
- BPAR
biopsy-proven acute rejection
- CNI
calcineurin inhibitor
- DCDD
donation after circulatory determination of death
- DI
depleting induction
- eGFR
estimated glomerular filtration rate
- FDA
Federal Drug Administration
- HCV
Hepatitis C virus
- ICC
intraclass correlation coefficient
- INR
international normalized ratio
- IQR
interquartile range
- IS
immunosuppression
- KT
kidney transplantation
- LT
liver transplantation
- NDI
non-depleting induction
- OR
odds ratio
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation.
Data Availability Statement
The data that support the findings of this study are openly available from the United Network for Organ Sharing.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of this article.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation 2009;9(Suppl 3):S1–155. [DOI] [PubMed] [Google Scholar]
- 2.Dharnidharka VR, Naik AS, Axelrod DA, Schnitzler MA, Zhang Z, Bae S, Segev DL, Brennan DC, Alhamad T, Ouseph R, Lam NN, Nazzal M, Randall H, Kasiske BL. Center practice drives variation in choice of US kidney transplant induction therapy: a retrospective analysis of contemporary practice. Transplantation International 2018;31(2):198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gharibi Z, Ayvaci MUS, Hahsler M, Giacoma T, Gaston RS, Tanriover B. Cost-effectiveness of antibody-based induction therapy in deceased donor kidney transplantation in the United States. Transplantation 2017;101(6):1234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyawala N, Silber JH, Rosenbaum PR, Wang W, Hill AS, Reiter JG, Niknam BA, Even-Shoshan O, Bloom RD, Sawinski D, Nazarian S, Trofe-Clark J, Lim MA, Schold JD, Reese PP. Comparing outcomes between antibody induction therapies in kidney transplantation. Journal of the American Society of Nephrology 2017;Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 5.Hill P, Cross NB, Barnett AN, Palmer SC, Webster AC. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database of Systematic Reviews 2017;1(CD004759). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briasoulis A, Inampudi C, Pala M, Asleh R, Alvarez P, Bhama J. Induction immunosuppressive therapy in cardiac transplantation: a systematic review and meta-analysis. Heart Failure Reviews 2018;23(5):641–9. [DOI] [PubMed] [Google Scholar]
- 7.Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, Meiser B, Rossano JW, Chambers DC, Yusen RD, Stehlik J, International Society for Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth Adult Heart Transplantation Report-2017; focus theme: allograft ischemic time. Journal of Heart and Lung Transplantation 2017;36(10):1037–46. [DOI] [PubMed] [Google Scholar]
- 8.Ansari D, Lund LH, Stehlik J, Andersson B, Hoglund P, Edwards L, Nilsson J. Induction with anti-thymocyte globulin in heart transplantation is associated with better long-term survival compared with basiliximab. Journal of Heart and Lung Transplantation 2015;34(10):1283–91. [DOI] [PubMed] [Google Scholar]
- 9.Duffy JSJ, Tumin D, Pope-Harman A, Whitson BA, Higgins RS, Hayes DJ. Induction therapy for lung transplantation in COPD: analysis of the UNOS registry. COPD 2016;13(5):647–52. [DOI] [PubMed] [Google Scholar]
- 10.Furuya Y, Jayarajan SN, Taghavi S, Cordova FC, Patel N, Shiose A, Leotta E, Criner GJ, Guy TS, Wheatley GH, Kaiser LR, Toyoda Y. The impact of alemtuzumab and basiliximab induction on patient survival and time to bronchiolitis obliterans syndrome in double lung transplantation recipients. American Journal of Transplantation 2016;16(8):2334–41. [DOI] [PubMed] [Google Scholar]
- 11.Kirkby S, Whitson BA, Wehr AM, Lehman AM, Higgins RS, Hayes DJ. Survival benefit of induction immunosuppression in cystic fibrosis lung transplant recipients. Journal of Cystic Fibrosis 2015;14(1):104–10. [DOI] [PubMed] [Google Scholar]
- 12.Hachem RR, Edwards LB, Yusen RD, Chakinala MM, Alexander Patterson G, Trulock EP. The impact of induction on survival after lung transplantation: an analysis of the International Society for Heart and Lung Transplantation Registry. Clinical Transplantation 2008;22(5):603–8. [DOI] [PubMed] [Google Scholar]
- 13.Cai J, Terasaki PI. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: an analysis of United Network for Organ Sharing registry data. Transplantation 2010;90(12):1511–5. [DOI] [PubMed] [Google Scholar]
- 14.Turner AP, Knechtle SJ. Induction immunosuppression in liver transplantation: a review. Transplant International 2013;26(7):673–83. [DOI] [PubMed] [Google Scholar]
- 15.Petite SE, Bollinger JE, Eghtesad B. Antithymocyte globulin induction therapy in liver transplant: old drug, new uses. The Annals of Pharmacotherapy 2016;50(7):592–8. [DOI] [PubMed] [Google Scholar]
- 16.Penninga L, Wettergren A, Wilson CH, Chan AW, Steinbruchel DA, Gluud C. Antibody induction versus placebo, no induction, or another type of antibody induction for liver transplant recipients. The Cochrane Database of Systematic Reviews 2014;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penninga L, Wettergren A, Wilson CH, Chan AW, Steinbruchel DA, Gluud C. Antibody induction versus corticosteroid induction for liver transplant recipients. Cochrane Database of Systematic Reviews 2014;31(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transplantation 2013;19(1):3–26. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food & Drug Administration. Thymoglobulin. 2018 Available from: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm089341.htm.
- 20.U.S. Food & Drug Administration. Package Insert: Simulect (basiliximab); Novartis Pharmaceutical Corp. 2003 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/basnov010203lb.htm#ind.
- 21.Electronic Medicines Compendium. Thymoglobuline 25mg powder for solution for infusion. 2016 Available from: https://www.medicines.org.uk/emc/product/6238/smpc.
- 22.European Medicines Agency. Simulect (basiliximab) 2005 Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/simulect.
- 23.Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2016 Annual Data Report: Liver. American Journal of Transplantation 2018;18(Suppl 1):172–253. [DOI] [PubMed] [Google Scholar]
- 24.Eichler HG, Abadie E, Breckenridge A, Flamion B, Gustafsson LL, Leufkens H, Rowland M, Schneider CK, Bloechl-Daum B. Bridging the efficacy-effectiveness gap: a regulator’s perspective on addressing variability of drug response. Nature Reviews Drug Discovery 2011;10(7):495–506. [DOI] [PubMed] [Google Scholar]
- 25.De Lusignan S, Crawford L, Munro N. Creating and using real-world evidence to answer questions about clinical effectiveness. Journal of Innovation in Health Informatics 2015;22(3):368–73. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on FDA’s new strategic framework to advance use of real-world evidence to support development of drugs and biologics. 2018 Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm627760.htm.
- 27.Corrigan-Curay J, Sacks L, Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA 2018;320(9):867–8. [DOI] [PubMed] [Google Scholar]
- 28.Nazzal M, Lentine KL, Naik AS, Ouseph R, Schnitzler MA, Zhang Z, Randall H, Dharnidharka VR, Segev DL, Kasiske BL, Hess GP, Alhamad T, McAdams-DeMarco M, Axelrod DA, Naik AS. Center-driven and clinically driven variation in U.S. liver transplant maintenance immunosuppression therapy: a national practice patterns analysis. Transplantation Direct 2018;4(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Axelrod DAN, A.S., Schnitzler MA, Segev DL, Dharnidharka VR, Brennan DC, Bae S, Chen J, Massie A, Lentine K. National variation in use of immunosuppression for kidney transplantation: a call for evidence-based regimen selection. American Journal of Transplantation 2016;16(8):2453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uemura T, Schaefer E, Hollenbeak CS, Khan A, Kadry Z. Outcome of induction immunosuppression for liver transplantation comparing anti-thymocyte globulin, daclizumab, and corticosteroid. Transplantation International 2011;24(7):640–50. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of Internal Medicine 2006;145(4):247–54. [DOI] [PubMed] [Google Scholar]
- 32.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20(4):488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levitsky J, Goldberg DS, Smith AR, Mansfield SA, Gillespie BW, Merion RM, Lok AS, Levy G, Kulik L, Abecassis M, Shaked A. Acute rejection increases the risk of graft failure and death in recent liver transplant recipients. Clinical Gastroenterology and Hepatology 2017;15(4):584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu S, Crespi CM, Wong WK. Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemporary Clinical Trials 2012;33(5):869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. Journal of Hepatology 2016;64(2):433–85. [DOI] [PubMed] [Google Scholar]
- 36.Tan PS, Muthiah MD, Koh T, Teoh YL, Chan A, Kow A, Zheng Q, Kwon CHD, Lee GH, Lesmana CRA, Villa V, Fung J, Lim K. Asian Liver Transplant Network clinical guidelines on immunosuppression in liver transplantation. Transplantation 2018;Epub ahead of print. [DOI] [PubMed]
- 37.Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H, Rostaing L, Rimola A, Marshall S, Mayer AD, Group. RS. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the ‘ReSpECT’ study. American Journal of Transplantation 2009;9(2):327–36. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida EM, Marotta PJ, Greig PD, Kneteman NM, Marleau D, Cantarovich M, Peltekian KM, Lilly LB, Scudamore CH, Bain VG, Wall WJ, Roy A, Balshaw RF, Barkun JS. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low-dose tacrolimus regimen vs. a standard-dose tacrolimus and mycophenolate mofetil regimen: a multicenter randomized clinical trial. Liver Transplantation 2005;11(9):1064–72. [DOI] [PubMed] [Google Scholar]
- 39.Lin CC, Chuang FR, Lee CH, Wang CC, Chen YS, Liu YW, Jawan B, Chen CL. The renal-sparing efficacy of basiliximab in adult living donor liver transplantation. Liver Transplantation 2005;11(10):1258–64. [DOI] [PubMed] [Google Scholar]
- 40.Goralczyk AD, Hauke N, Bari N, Tsui TY, Lorf T, Obed A. Interleukin 2 receptor antagonists for liver transplant recipients: a systematic review and meta-analysis of controlled studies. Hepatology 2011;54(2):541–54. [DOI] [PubMed] [Google Scholar]
- 41.Lexicon Plus - Cerner Multum [Internet]. Cerner Corporation 2016. [cited May 13, 2019]. Available from: https://www.cerner.com/solutions/drug-database.
- 42.Simon DM, Levin S. Infectious complications of solid organ transplantations. Infectious Diseases Clinics of North America 2001;15(2):521–49. [DOI] [PubMed] [Google Scholar]
- 43.Taylor AN, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Critical Reviews in Oncology/Hematology 2005;56(1):155–67. [DOI] [PubMed] [Google Scholar]
- 44.Jones-Hughes T, Snowsill T, Haasova M, Coelho H, Crathorne L, Cooper C, Bujica-Mota R, Peters J, Varley-Campbell J, Huxley N, Moore J, Allwood M, Lowe J, Hyde C, Hoyle M, Bond M, Anderson R. Immunosuppressive therapy for kidney transplantation in adults: a systematic review and economic model. Health Technology Assessment 2016;20(62):1–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cremaschi L, von Versen R, Benzing T, Wiesener M, Zink N, Milkovich G, Paivanas T, Gallagher M, Thaiss F. Induction therapy with rabbit antithymocyte globulin versus basiliximab after kidney transplantation: a health economic analysis from a German perspective. Transplant International 2017;30(10):1011–9. [DOI] [PubMed] [Google Scholar]
- 46.Gras JM, Gerkens S, Beguin C, Janssen M, Smets F, Otte JB, Sokal E, Reding R. Steroid-free, tacrolimus-basiliximab immunosuppression in pediatric liver transplantation: clinical and pharmacoeconomic study in 50 children. Liver Transplantation 2008;14(4):469–77. [DOI] [PubMed] [Google Scholar]
- 47.Plasencia-Garcia I, Tevar-Alfonso E, Gonzalez-Rodriguez A, Moreno-Garcia A, Merino-Alonso J, Aguirre-Jaime A. Clinical-economic impact of the change of protocol for use of basiliximab in liver transplant. Farmacia Hospitalaria 2019;43(1):13–8. [DOI] [PubMed] [Google Scholar]
- 48.Dopazo C, Charco R, Caralt M, Pando E, Lazaro JL, Gomez-Gavara C, Castells L, Bilbao I. Low total dose of anti-human T-lymphocyte globulin (ATG) guarantees a good glomerular filtration rate after liver transplant in recipients with pretransplant renal dysfunction. Canadian Journal of Gastroenterology and Hepatology 2018;eCollection 2018(1672621). [DOI] [PMC free article] [PubMed]
- 49.Peters SA, Bots ML, Den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR, O’Leary DH, Evans GW, Raichlen JS, Moons KG, Koffijberg H, METEOR study group. Multiple imputation of missing repeated outcome measurements did not add to linear mixed-effects models. Journal of Clinical Epidemiology 2012;65(6):686–95. [DOI] [PubMed] [Google Scholar]
- 50.Little RJA, Rubin DB. Statistical Analysis with Missing Data 2nd ed: Wiley; 2002. [Google Scholar]
- 51.Mangus RS, Lutz AJ, Fridell JA, Kubal CA, Bush WJ, Tector AJ. Minimal improvement in glomerular filtration rate in the first year after liver transplantation. Transplantation 2015;99(9):1855–61. [DOI] [PubMed] [Google Scholar]
- 52.Israni AK, Xiong H, Liu J, Salkowski N, Trotter JF, Snyder JJ, Kasiske BL. Predicting end-stage renal disease after liver transplant. American Journal of Transplantation 2013;13(7):1782–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.