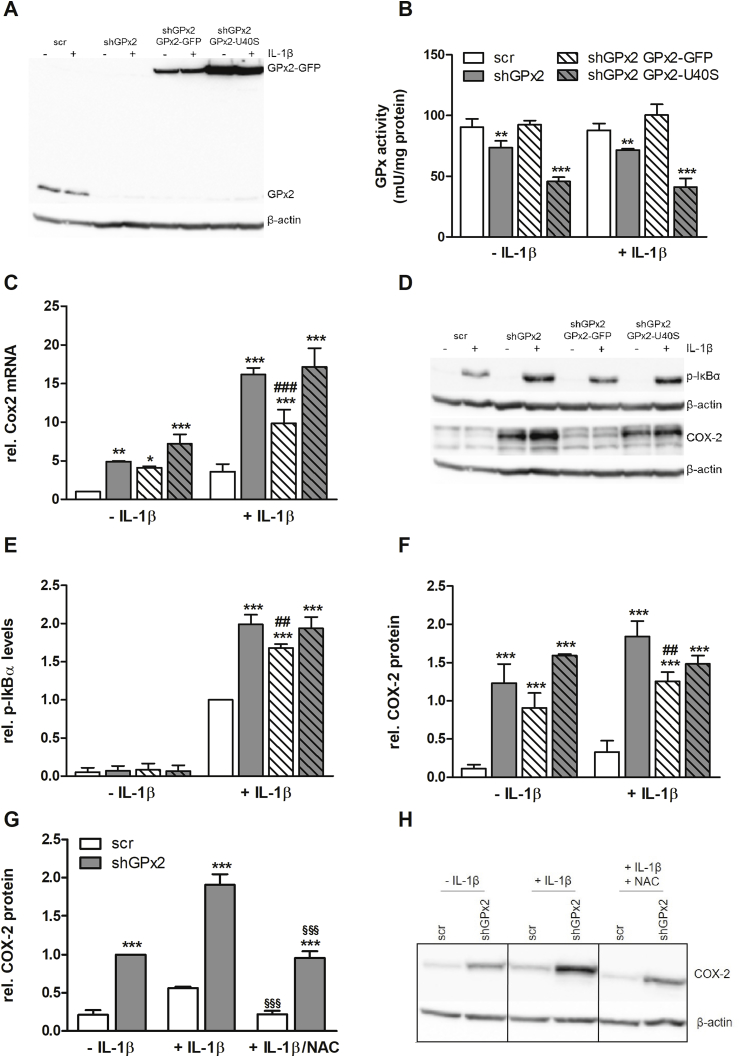
Fig. 2.
Suppression of pro-inflammatory signal transduction requires redox-active GPx2. Stable GPx2 kd HT-29 cells (shGPx2) were further transfected with a non-targetable GFP-tagged GPx2 (shGPx2 GPx2-GFP) or with a GFP-tagged mutant form of GPx2 (shGPx2 GPx2-U40S). After supplementation with 50 nM sodium selenite for 72 h, endogenous GPx2 and GFP-tagged GPx2 expression was analyzed by Western blot 24 h after stimulation with 1 ng/mL IL-1β (A). In addition, GPx activity was spectrophotometrically determined using H2O2 as substrate (B). Cells were stimulated for 3 h with IL-1β and transcript levels of COX-2 were measured by qPCR and normalized to Rpl13a and Oaz1 (C). In the same lysates used in (A), COX-2 protein levels were detected by Western Blot (D, F). Cells were stimulated for 1 h with IL-1β and p-IκBα protein levels were detected by Western Blot (D, E). Cells were stimulated for 4 h with IL-1β with or without pretreatment for 1 h with 50 mM NAC, and COX-2 protein levels were measured by Western blot (G and H). Western blot bands were normalized to β-actin. Data are given as means + SD (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001 vs. respective scr; #p < 0.05; ##p < 0.01; ###p < 0.001 vs. shGPx2 and §§§p < 0.001 vs. +IL-1β analyzed by two-way ANOVA with Bonferroni's post-test.