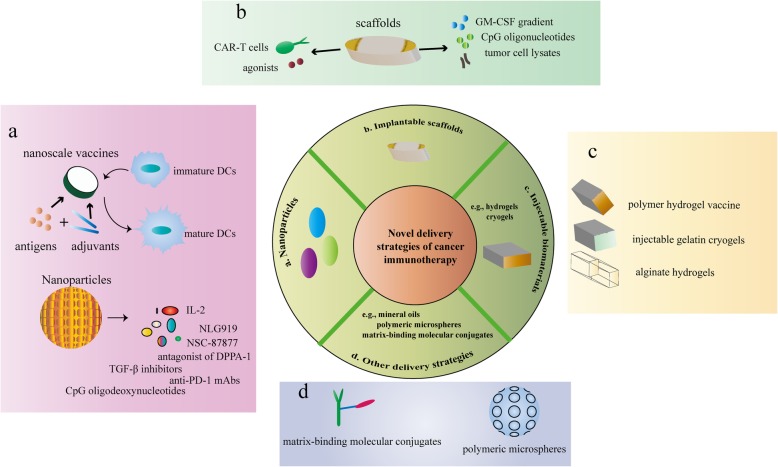
Fig. 2.
Novel delivery strategies of immunotherapy with improved efficacy and safety. a Nanoparticle-based delivery of immunotherapy. Nanoparticles can mediate the delivery of vaccines. The most researched nanoscale vaccines were antigen (e.g., proteins and peptides)-TLR agonist fusion vaccines. Amphiphilic nanoscale vaccines have also been created which are composed of antigen or adjuvant cargo attached to the tail of the lipophilic albumin. High-density lipoprotein mimic nanodiscs conjugated to neoantigen peptides and adjuvants were developed. Nanodisc-based vaccines can greatly increase the efficiency of co-delivery of antigens and adjuvants to lymphoid tissues and thus induce DCs maturation. Moreover, nanoparticle-mediated delivery targets multiple inhibitory signals in the tumor microenvironment. Therapeutic peptide assembly nanoparticles, an antagonist of d-peptide programmed cell death ligand 1 (DPPA-1), were fabricated and co-assembled with NLG919 (an inhibitor of indoleamine 2,3-dioxygenase 1 (IDO-1)). In addition, nanoscale liposome polymer gels (nLGs), including TGF-β inhibitors and IL-2, were designed. And nano-cocoons can control the release of anti-PD-1 antibodies and CpG oligodeoxynucleotides, which can prevent cancer recurrence and prolong mouse survival. NSC-87877, a potent Shp1 and shp2 protein tyrosine phosphatases inhibitor, was packaged in the nanoparticles. Nanoparticles carrying NSC-87877 were conjugated to the surface of tumor-specific T cells and stimulated T cell expansion. b Implantable scaffolds for the delivery of immunotherapy. Implantable scaffolds are biomaterials that can be preloaded with a variety of chemical reagents, biological factors, or cells. The scaffolds are typically implanted through a small surgical procedure into the subcutaneous or resected sites. The bioactive agents can be controlled to release in the implanted scaffold, and the immune cells are typically recruited to access the scaffolds for further bio-programming. For example, poly (lactide-co-glycolide) (PLG) polymer scaffolds were designed to contain GM-CSF, CpG oligonucleotides, and tumor cell lysates as recruitment factors, risk signals, and antigen sources, respectively. Alginate scaffolds can co-deliver CAR-T cells with cyclic dinucleotide (CDN) STING agonists to treat solid tumors. c Injectable biomaterials for immunotherapy. Injectable biomaterials include hydrogels and cryogels. The advantage of these materials is that they can be positioned anywhere the needle can reach without the need for surgical implantation. This is a relatively simple and minimally invasive procedure that does not require much technical expertise and avoids unnecessary tissue damage and a series of complications related with inflammatory wound response. d. Other delivery strategies: matrix-binding molecular conjugates, mineral oils, and polymeric microspheres. Matrix-binding molecular conjugates have been developed to accumulate within and around tumors, reducing systemic drug exposures and side effects. For example, with a water-soluble amine-sulfhydryl crosslinker, checkpoint inhibitors bound to a peptide from placental growth factor 2 (PLGF2), which has a particularly high affinity for a variety of matrix proteins. These conjugates were more localized in the extracellular matrix around the tumor tissue, leading to delayed tumor growth and extended survival. Mineral oils and polymeric microspheres are designed for local and controlled release. A commercially available light mineral oil blend, Montanide ISA 51, has been applied in clinical trials for immunotherapy. This mixture was utilized to prepare sustained release formulations that delivered agonistic anti-CD40 antibodies locally. In addition, biodegradable polymer microparticle formulations have also been developed to deliver immunomodulatory antibodies locally and continuously, including PLHMGA