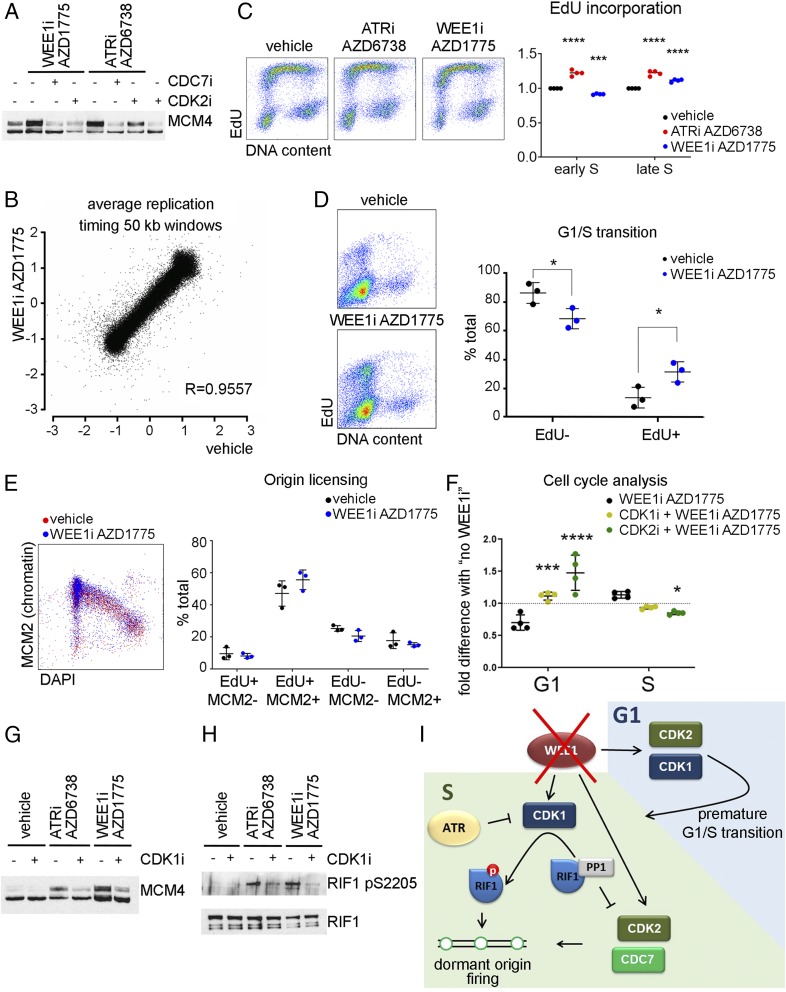
Fig. 1.
(A) The 293T cells were pretreated with vehicle (dimethyl sulfoxide), 10 µM CDK2i, or 10 µM CDC7i for 15 min before 5 µM ATRi or 500 nM WEE1i for 30 min. Immunoblot of the nuclease-insoluble fraction. (B) U2OS cells were treated with WEE1i for 60 min, and 5-bromodeoxyuridine was added for the last 30 min. Repli-Seq correlation between log ratio of early S to late S at LOESS smoothing step of 50-kb windows along the genome. R, Pearson correlation coefficient. (C) U2OS cells were treated with ATRi or WEE1i for 45 min, and 10 µM EdU was added for the last 15 min. Cells were fixed with ethanol and stained for EdU and DNA. (Left) Flow cytometry plots. (Right) Quantification of 4 independent experiments. (D). U2OS cells were synchronized by thymidine–nocodazole block. Three hours postrelease, WEE1i was added for 45 min, and EdU was added for the last 15 min. (Left) Flow cytometry plots. (Right) Quantification of 3 independent experiments. (E) U2OS cells were treated with WEE1i for 45 min, and EdU was added for the last 15 min. After extraction, cells were fixed and stained for chromatin-bound MCM2, EdU, and DNA content (DAPI). (Left) Flow cytometry plots. (Right) Quantification of 3 independent experiments. (F) U2OS cells were pretreated with 5 µM CDK1 or 5 µM CDK2 inhibitors for 15 min before the addition of WEE1i for 45 min, and EdU was added for the last 15 min. The effect of WEE1i on the percent of G1- or S-phase cells (normalized to samples with no WEE1i) is shown (4 independent experiments). (G) The 293T cells were pretreated with 5 µM CDK1i for 15 min before the addition of ATRi or WEE1i for 30 min. Immunoblot of the nuclease insoluble fraction. (H) The 293T cells were transfected with Rif1-GFP. Forty-eight hours after transfection, cells were pretreated with CDK1i for 15 min before the addition of ATRi or WEE1i for 1 h. RIF1 was immunoprecipitated using green fluorescent protein trap beads. (I) Cartoon depicting the roles of WEE1 in controlling replication initiation. *P < 0.05, ***P < 0.0005, ****P < 0.0001.