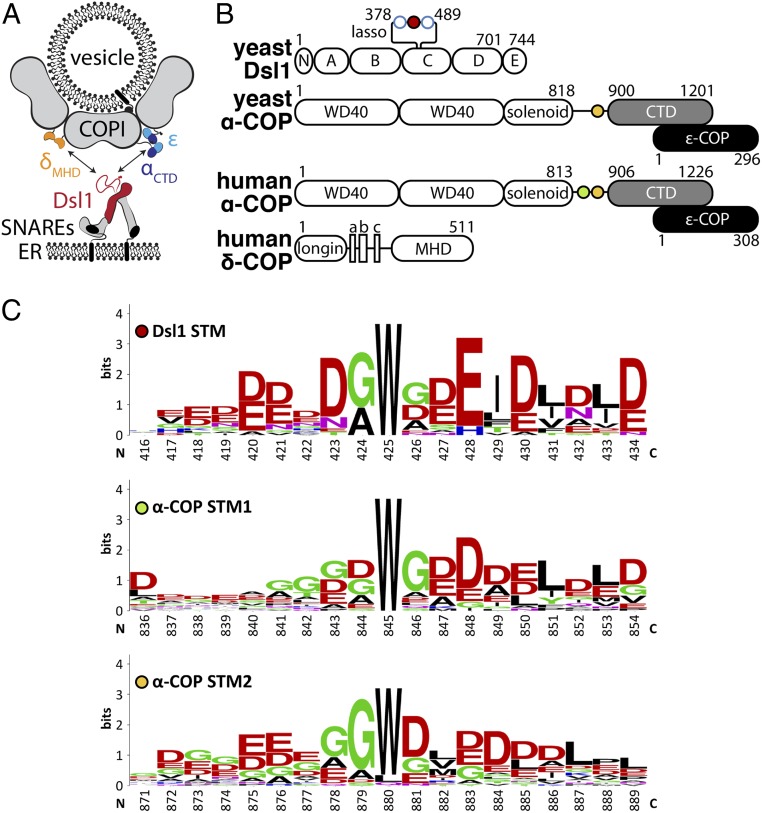
Fig. 1.
STM sequences in Dsl1 and α-COP. (A) The Dsl1 tethering factor binds to ER-localized SNAREs (Bottom) and, via its Dsl1 subunit (red), to 2 domains of the COPI vesicle coat, αCTD (blue) and δMHD (orange). (B) The domain structures of the proteins in this study are shown schematically. The C-terminal domain (CTD) of α-COP (gray) forms a stable complex with ε-COP (black). Singleton tryptophan motifs (STMs) are depicted as filled circles, while open blue circles represent di-tryptophan motifs (see SI Appendix, Fig. S1A for details). (C) Sequence logos of STMs found in Dsl1 and α-COP (full aligned sequences are shown in SI Appendix, Fig. S1). Dsl1 numbering is relative to S. cerevisiae, whereas α-COP numbering is relative to Homo sapiens.