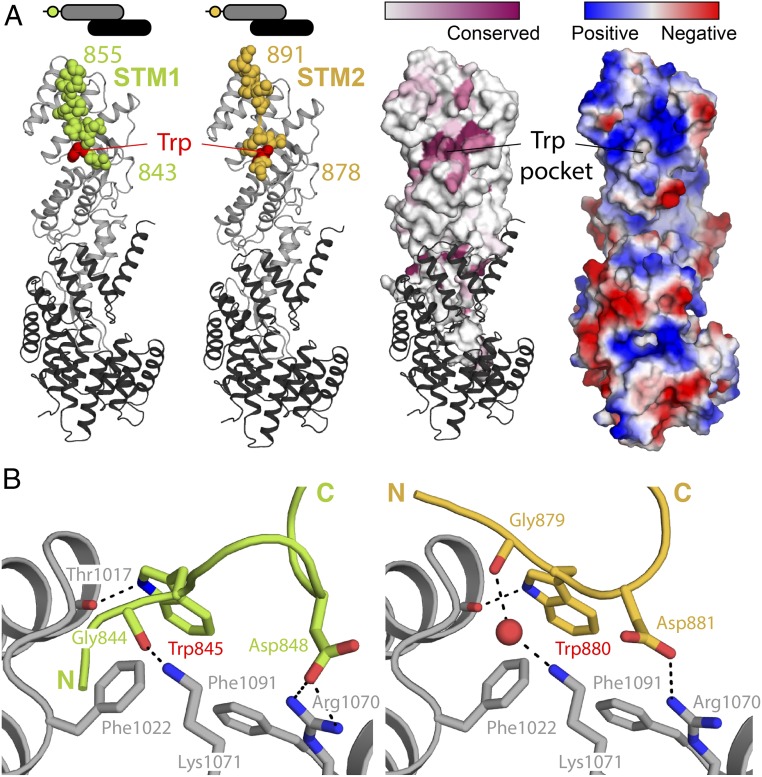
Fig. 2.
Structural basis of STM1/STM2 recognition by human αCTD•ε. (A) Crystal structures of human αSTM1-CTD•ε (Left) and αSTM2-CTD•ε (Right). The αCTD•ε heterodimer is oriented with αCTD (gray) at the Top and ε-COP (black) at the Bottom. In each case, the STM (spheres) belongs to a symmetry-related molecule (see text and Fig. 3A). On the Right, the surface of αCTD is colored by amino acid conservation or by electrostatic potential, highlighting the conserved, nonpolar cavity that accommodates the singleton tryptophan residue. (B) αCTD binds STM1 and STM2 through a combination of nonpolar interactions, hydrogen bonds, and salt bridges. The side chains of the STM1 and STM2 singleton tryptophan residues are superimposable within experimental error.