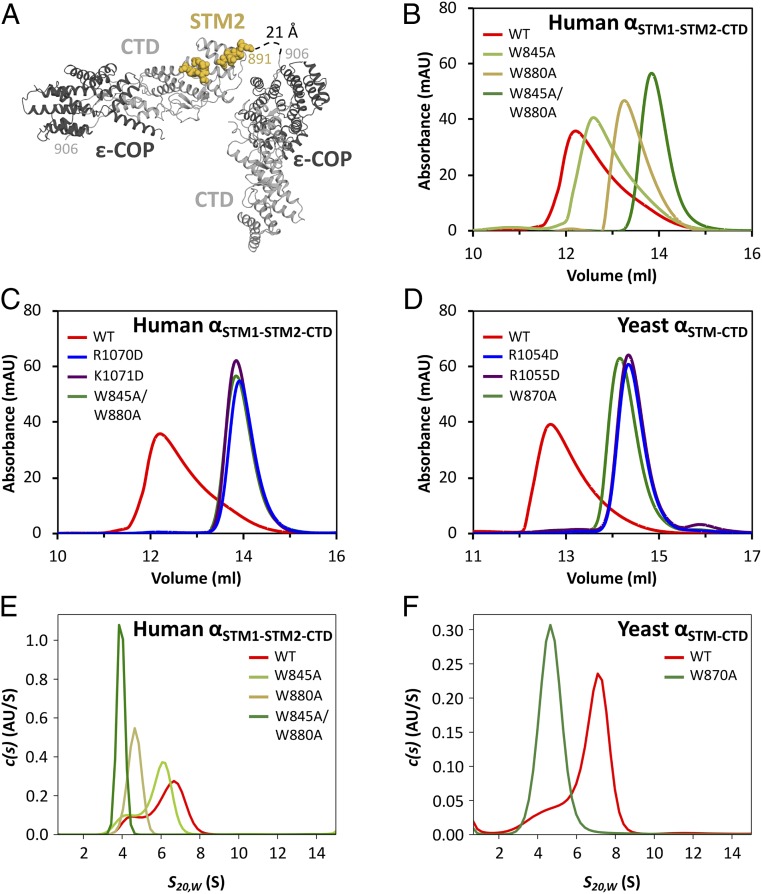
Fig. 3.
The STM•CTD interaction drives α-COP homo-oligomerization. (A) In the human αSTM2-CTD•ε crystal structure, the bound STM sequence is contributed by a symmetry-related molecule. (B) Size exclusion chromatography was used to analyze wild-type human αSTM1-STM2-CTD•ε, as well as mutants with one or both singleton tryptophans replaced with alanine. (C) Charge-reversal mutations in the STM binding site disrupt homo-oligomerization to the same extent as mutation of both singleton tryptophans. (D) Size exclusion chromatography was used to analyze wild-type yeast αSTM-CTD•ε, as well as the corresponding tryptophan replacement and charge reversal mutants. (E) Sedimentation velocity analytical ultracentrifugation of wild-type and mutant human αSTM1-STM2-CTD•ε confirmed that the STM•CTD interaction is required for homo-oligomerization. (F) Sedimentation velocity analytical ultracentrifugation of wild-type and mutant yeast αSTM-CTD•ε confirmed that the STM•CTD interaction is required for homo-oligomerization.