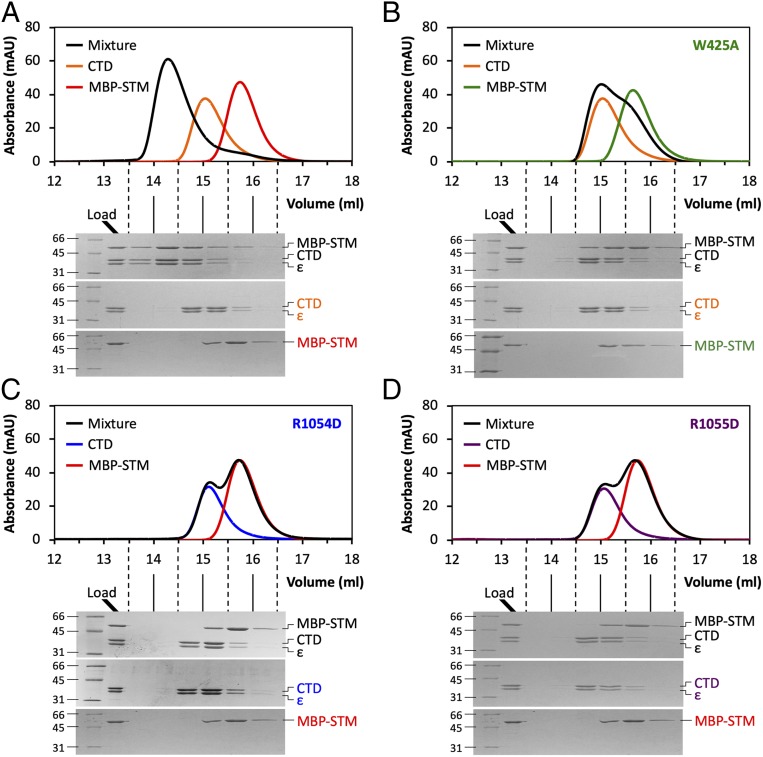
Fig. 5.
The Dsl1 lasso binds to the same conserved site on αCTD. (A) Size exclusion chromatography and SDS/PAGE demonstrate that yeast αCTD•ε forms a stable 1:1 complex with MBP-Dsl1410–440. In separate experiments, αCTD•ε, MBP-Dsl1410–440, or an equimolar mixture of the 2 (preincubated 1 h at 4 °C) was loaded onto the column. (B) Complex formation between yeast αCTD•ε and MBP-Dsl1410–440W425A is greatly diminished, as the elution profile of the mixture resembles the sum of the individual profiles. The data presented for αCTD•ε alone are identical to those presented in panel (A). (C) Complex formation between αCTDR1054D•ε and MBP-Dsl1410–440 is greatly diminished, as the elution profile of the mixture is indistinguishable from the sum of the individual profiles. The data presented for MBP-Dsl1410–440 alone are identical to those presented in panel (A). (D) As in C, but for αCTDR1055D•ε.