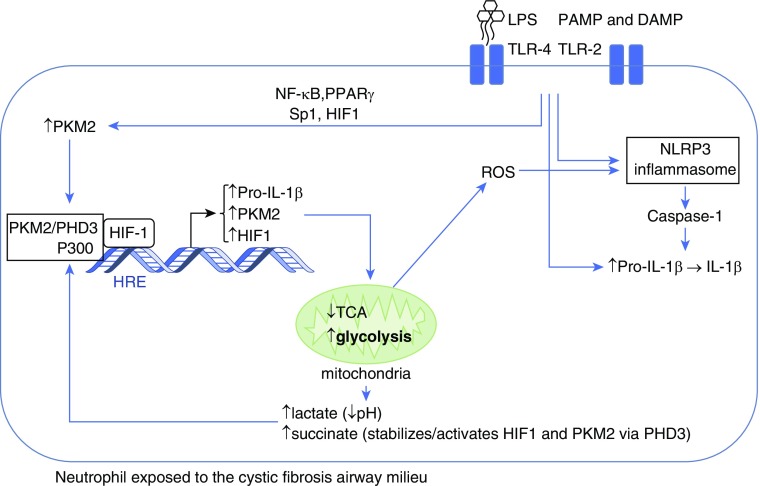
Figure 1.
Neutrophil immunometabolism in cystic fibrosis. On exposure to LPS in the cystic fibrosis airway, the neutrophil increases its cytoplasmic levels of the M2 isoform of PKM2 (pyruvate kinase) and pro–IL-1β. Simultaneously, the NLRP3 inflammasome is activated through several mechanisms. PKM2 in association with PHD3 (prolyl hydroxylase-3) is stabilized and in conjunction with the coactivator P300 facilitates HIF1 (hypoxia inducible factor-1)–dependent transcription that further increases the synthesis of pro–IL-1β, PKM2, and HIF1, while reprogramming mitochondria to deviate glucose from the tricarboxylic acid cycle (TCA) to the glycolytic pathway. Increased glycolysis results in production of lactate, acidification of the cytoplasm, and accumulation of succinate, which helps stabilize the HIF1 complex. Mitochondria release reactive oxygen species (ROS) that further activate the NLRP3 inflammasome, which converts pro–IL-1β to active IL-1β through caspase-1–mediated cleavage. DAMP = damage-associated molecular patterns; NF-κB = nuclear factor-κB; PAMP = pathogen-associated molecular patterns; PPARγ = peroxisome proliferator-activated receptor-γ; TLR = toll-like receptors.