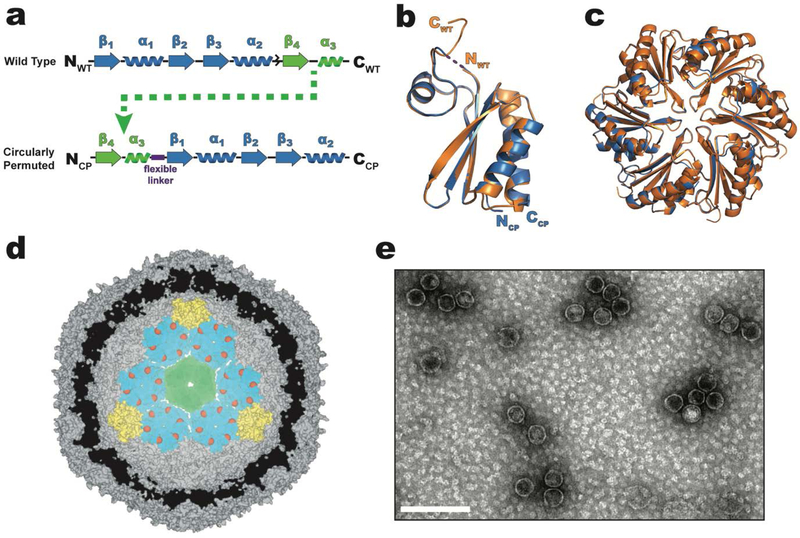
Figure 1.
Design and structure of a circularly permuted CPH protein that substitutes for the WTH in intact HO shells. a) primary structure of WTH (top) and circularly permuted CPH (bottom). The flexible (Gly-Ser)3 linker used to join the original NFWT- and CWT- termini of WTH is represented as a purple box. b) Structural alignment of WTH (orange, pdb 5djb) and CPH (blue, pdb 6nlu) protomers. No electron density was observed for the Gly-Ser linker and is therefore drawn as a dashed line. c) Alignment of the hexameric assemblies of WTH (orange) and CPH (blue). View is top-down looking at the convex side of the hexamers. d) Cutaway model (derived from pdb 5v74) of a minimal shell made of CPH (teal), T1 (green), and PSII (yellow) proteins showing the predicted luminal orientation of the CPH C-termini (red spheres). e) TEM micrograph of purified CPH-T1-PSII shells. Scale bar is 100 nm.