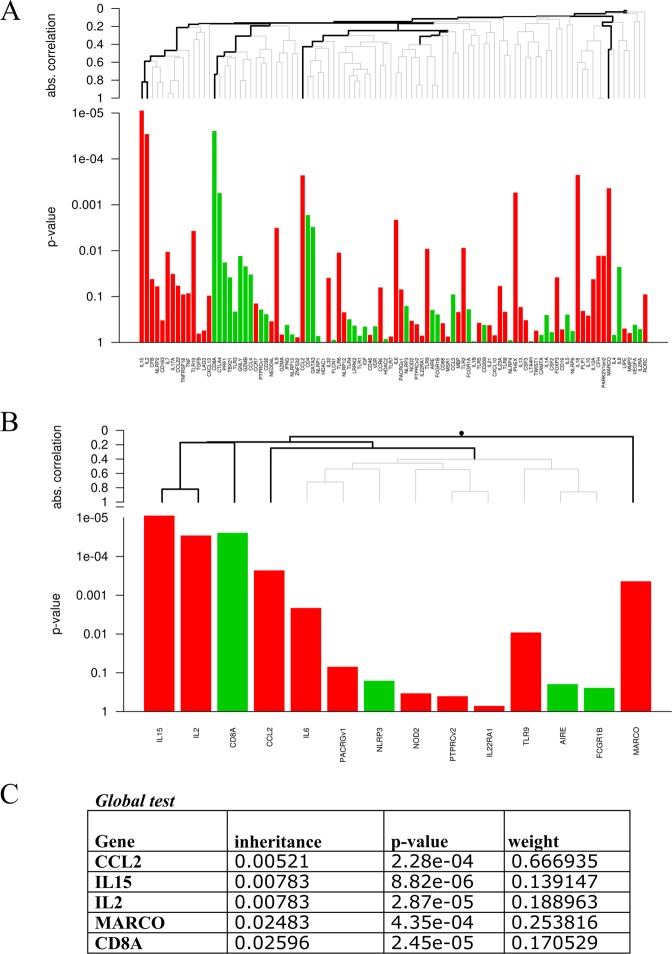
Figure 4.
Identification of a minimal biomarker risk signature for developing reversal reactions. Biomarker signature to assess the risk of BL/LL patients to develop reversal reactions (RR). Gene expression data obtained by dual-color RT-MLPA of RNA isolated from whole blood of BL/LL patients from Bangladesh, Brazil, Ethiopia and Nepal at t = 0 were analyzed using the global test cluster analysis41. The global test is a cluster analysis based on absolute correlation difference and average linkage developed for data sets in which many covariates (or features) have been measured for the same subjects, together with a response variable. Graphs (A,B) indicate genes that are higher expressed in future RR patients (red) or in non-reactional BL/LL patients (green). In (A) all genes analyzed are shown and in (B) only significant branches are shown. Table (C) shows values for the 5 genes that were statistically significant after correction for multiple testing (inheritance <0.05), representing the output signature of the global test shrinkage model.