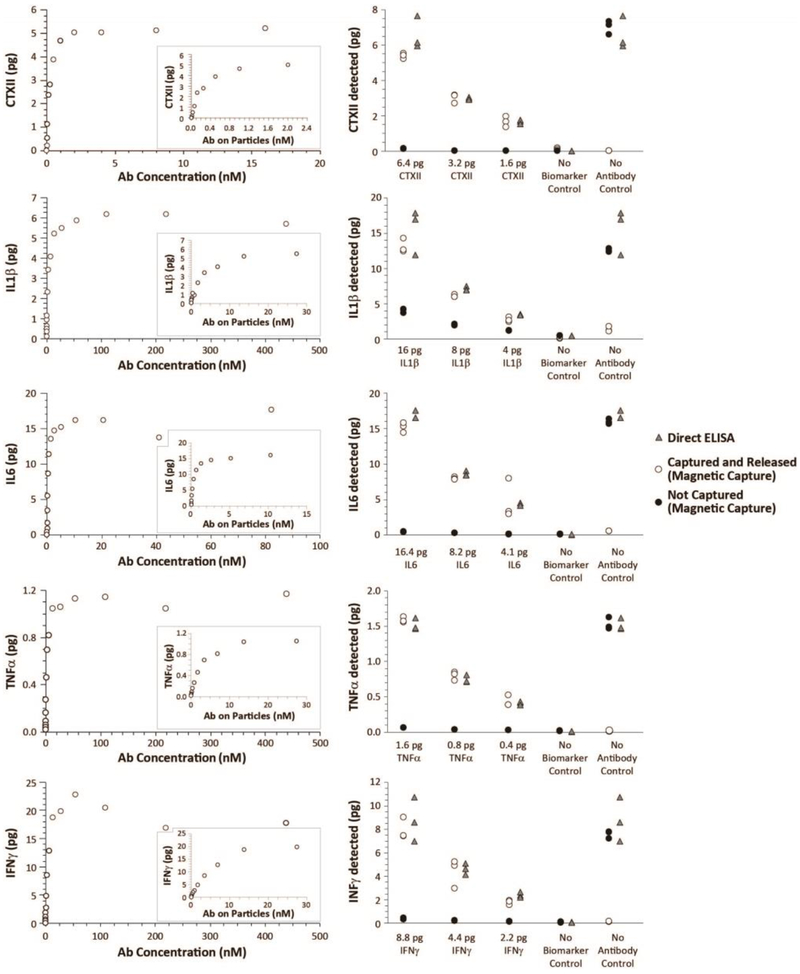
Figure 1: Proof-of-principle and validation of multiplex magnetic capture.
As a validation of multiplex magnetic capture, two experiments were designed (See Supplemental Figure 2). First, 0.6 pg/μL CTXII, 2.0 pg/μL IL1β, 2.1 pg/μL IL6, 0.2 pg/μL TNFα, and 1.1 pg/μL IFNγ were blended together in a biomarker blend. Then, increasing concentrations of antibody-conjugated particles (and thereby increasing concentrations of antibody) were added to biomarker blend. As particle concentrations increased, biomarker was depleted from the sample, as demonstrated by the plateauing collection curve (Left Column). Moreover, pH treatment was able to release the biomarker for quantification. Second, to validate magnetic capture relative to direct ELISA, a second biomarker blend was created (0.8 pg/μL CTXII, 2.0 pg/μL IL1β, 2.1 pg/μL IL6, 0.2 pg/μL TNFα, and 1.1 pg/μL IFNγ). This biomarker blend was then diluted 2- or 4-times. Antibody-conjugated particles were added to 8 μL of each biomarker blend (n=3 per biomarker blend); capture buffer without biomarkers (no biomarker control) and bare particles (no antibody control) were used as controls. Direct ELISAs were conducted on the same samples as a positive control. As shown in the Right Column, a multiplex magnetic capture was able to simultaneously assay CTXII, IL1β, IL6, TNFα, and IFNγ. While minor losses were observed in IL1β and IL6, these losses were stable and predictable across the different concentrations assessed. Combined, these data demonstrate these molecular targets can be collected, released, and assayed via magnetic capture.