Abstract
BACKGROUND
Both canola and sesame oils consumption have been associated with favorable effects on cardio-metabolic biomarkers. However, to the best of our knowledge, no study has compared their effects on cardiovascular risk factors. The present study aimed to assess the effect of canola, sesame, and sesame-canola oils consumption on cardio-metabolic biomarkers in patients with type 2 diabetes mellitus (T2DM).
METHODS
This study was a randomized, triple-blind, three-way, crossover clinical trial. The study participants included 102 individuals with T2DM. Their spouses were also included in the study. The participants were entered into a 4-week run-in period. After that, their regular dietary oil was replaced with canola, sesame, or sesame-canola oils (a blend of sesame and canola oils) in three 9-week phases, which were separated by two 4-week washout periods (sunflower oil was consumed during the run-in and the washout periods). Dietary, physical activity, blood pressure, and anthropometric measurements were assessed at the beginning, in the middle (week 4-5), and at the end of each treatment phase. Blood samples were taken at the beginning and at the end of each phase. Serum, plasma, buffy coat, and whole blood samples were extracted and kept at -80 ºC for further analysis. Serum fasting blood sugar (FBS), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were selected as the primary outcomes.
RESULTS
102 participants with T2DM were randomly assigned to one of the 6 rolling methods. Through them, 93 individuals (91.2%) completely participated in all phases.
CONCLUSION
The present study will provide an exceptional opportunity to examine the effect of canola, sesame, and sesame-canola oil on cardio-metabolic markers in adults with and without T2DM. This trial will also provide a good medium for the investigation of gene-dietary oils interaction in the future.
Keywords: Canola Oil, Sesame Oil, Cardiovascular Diseases, Type 2 Diabetes Mellitus, Clinical Trial
Introduction
The replacement of saturated fatty acids with polyunsaturated fatty acids (PUFAs) in a diet have led to a decrease in the risk of chronic diseases like type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD).1,2 Moreover, it has been reported that PUFAs consumption has several beneficial consequences for human health.3-5 In addition, linoleic acid, which is the most abundant omega-6 PUFA, is associated with decreased T2DM risk,6 and may improve cholesterol and insulin sensitivity status.7 Furthermore, omega-3 PUFAs, especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) might improve lipid profile,8,9 and consequently, the risk of CVD.10,11
Canola oil (CO) is proposed as a good source of PUFAs, including linoleic acid, mono-unsaturated fatty acids (MUFAs), and alpha-linolenic acid (ALA), an omega-3 fatty acid that can be converted to DHA and EPA in the human body.12 It has been suggested that CO intake might improve serum total cholesterol (TC),13 low-density lipoprotein cholesterol (LDL-C),14,15 apolipoprotein B to apolipoprotein A1 ratio (Apo B/Apo A1),15 and triglyceride (TG)14,16 levels. Additionally, some studies found that CO consumption decreased circulating levels of fasting glucose14-16 and insulin13,16, while some other studies could not find the same results.13,17,18 In contrast, sesame oil (SO) contains high amounts of omega-6 PUFAs and MUFAs19 such as linoleic and oleic acid, respectively.20 Furthermore, SO contains significant amounts of antioxidant phytochemicals including sesamin, sesamolin, sesaminol,21,22 and vitamin E.19 Sesamin might have anti-atherosclerotic properties23 and might help to control hypertension.19,24 In patients with insulin resistance, SO consumption resulted in a significant reduction in serum TC and LDL-C level with no significant effect on TG.25 However, in a study on patients with diabetes, SO improved not only plasma glucose, TC, and LDL-C, but also TG levels.19
To the best of our knowledge, a limited number of high-quality trials have examined the effect of CO and SO on cardio-metabolic markers, which have led to inconsistent results. Moreover, no study has compared the effect of SO with that of CO, which are considered as healthy edible oils. CO is one of the largest sources of edible oils consumed worldwide, and SO has been regarded as a healthy oil in Asian countries for a long time.19 It is also noteworthy that adults with T2DM experience several metabolic abnormalities particularly in terms of insulin sensitivity, blood glucose levels, and lipid profile, which independently lead to a higher risk of serious disease including CVD.26 Therefore, the present clinical trial was conducted to assess the effect of SO compared with CO and sesame-canola oil (SCO: a blend of these two edible oils) on cardio-metabolic markers, including lipid profile, glycemic indices, blood pressure, and anthropometric measurements in adults with T2DM and their spouses by replacing participants’ regular consumed oils with the mentioned oils.
Materials and Methods
Trial design and setting: This study was a randomized, triple-blind, three-way crossover, clinical trial which aimed to assess the effect of replacing regular oil consumption of adults with T2DM with SO, CO, and SCO on cardio-metabolic markers. The patients’ spouses were also included in the present study and received all the interventions because we aimed to replace the oils regularly used at home with the abovementioned oils. The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) was used as a framework for reporting the present protocol.27
The medical records of individuals referred to Diabetes Research Center of Shahid Sadoughi University of Medical Sciences, Yazd, Iran, were reviewed to identify potential participants based on the eligibility criteria. In the initial visit, after explaining the study procedure to the participants and obtaining their informed consents and medical history, the participants' demographic information and medication use were recorded, and a 24-hour food recall and a 24-hour physical activity recall were completed for the participants. Body composition, anthropometric, and blood pressure measurements were also performed on the first visit by a trained nutritionist. Moreover, the daily energy requirement of the participants and their spouses were estimated using formulas suggested by the US Institute of Medicine (IoM).28 Thereafter, they received a healthy dietary recommendation, which provided 30-32% of the total energy needs from fats, 50-52% from carbohydrates, and 16-18% from proteins. The study subjects were recommended to maintain their physical activity throughout the study period. Additionally, nutrition counseling was provided by a trained nutritionist.
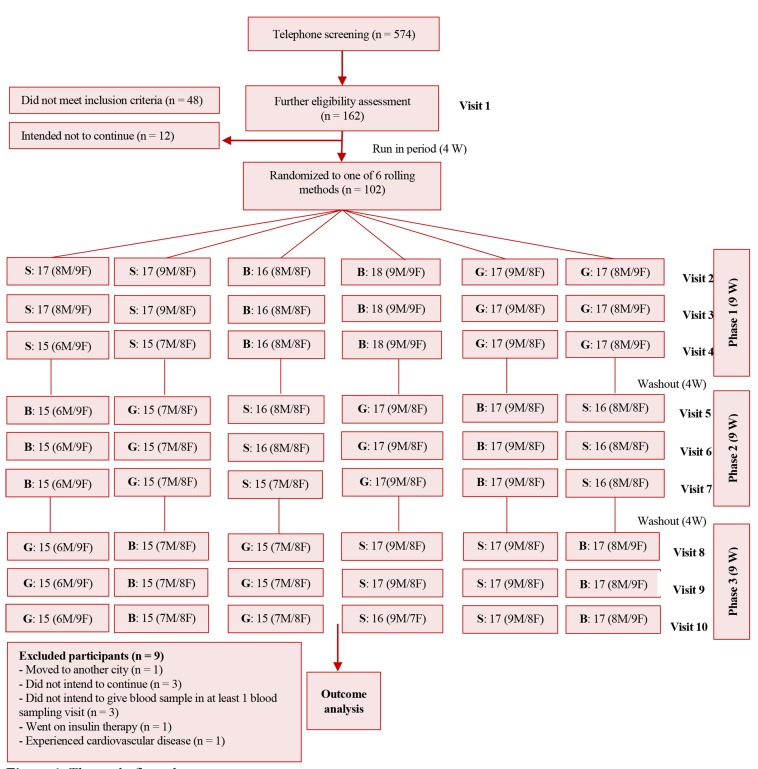
After the first visit, participants and their spouses were entered into a 4-week run-in period in which their regular consumed oils were replaced with sunflower oil. The intervention oils were provided in the same packages, which were labeled with three codes (B, G, and S), and individuals were randomly assigned to consume them. Each intervention period lasted 9 weeks and 4-week intervals (sunflower oil was provided) separated the intervention periods as washout durations. The study flow diagram is presented in figure 1. The oils were provided for the study participants and their family by investigators.
Figure 1.
The study flow chart
Participants were randomized to six rolling methods to receive canola oil, sesame oil, and sesame-canola oil with three codes (B, G and S). F: Female, M: Male, W: Weeks
There were three clinical visits at the beginning, in the middle (forth to fifth week), and at the end of each intervention period. The details of all measurements conducted in each visit are provided in table 1. All measurements and blood samplings were also performed for the participants’ spouses.
Table 1.
Details of the study visitsλ
| Measured variable | Phase 1 | Phase 2 | Phase 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2** | Visit 3*** | Visit 4£ | Visit 5** | Visit 6*** | Visit 7£ | Visit 8** | Visit 9*** | Visit 10£ | ||
| Eligibility criteria assessment | * | ||||||||||
| Medical history | * | ||||||||||
| Informed consent | * | ||||||||||
| Nutrition counseling | * | ||||||||||
| Medication use | * | * | * | * | * | * | * | * | * | * | |
| Physical activity | * | * | * | * | * | * | * | * | * | * | |
| 24-hour dietary recall | * | ||||||||||
| Anthropometric measurements | |||||||||||
| Weight | * | * | * | * | * | * | * | * | * | * | |
| Height | * | ||||||||||
| Waist circumference | * | * | * | * | * | * | * | * | * | * | |
| Hip circumference | * | * | * | * | * | * | * | * | * | * | |
| Body composition indices | |||||||||||
| Body fat mass | * | * | * | * | * | * | * | * | * | * | |
| Lean mass | * | * | * | * | * | * | * | * | * | * | |
| Visceral fat | * | * | * | * | * | * | * | * | * | * | |
| Blood pressure | * | * | * | * | * | * | * | * | * | * | |
| Blood sampling | * | * | * | * | * | * | |||||
| Biochemical assessments | |||||||||||
| FBS | * | * | * | * | * | * | |||||
| TG | * | * | * | * | * | * | |||||
| TC | * | * | * | * | * | * | |||||
| HDL-C | * | * | * | * | * | * | |||||
| LDL-C | * | * | * | * | * | * | |||||
| Apo A | * | * | * | * | * | * | |||||
| Apo B | * | * | * | * | * | * | |||||
| Lp (a) | * | * | * | * | * | * | |||||
| Capillary fasting blood glucose | * | * | * | ||||||||
| Compliance | |||||||||||
| Three-day food records | * | * | * | * | * | * | * | * | * | ||
| Weight measurement of provided oils | * | * | * | * | * | * | * | * | * | * | |
All assessments except plasma FFAs profile will be assessed for the participants and their spouses;
Visit at the beginning of the intervention phases;
Visit in the middle of intervention phases;
Visit at the end of intervention phases;
FBS: Fasting blood sugar; TG: Triglyceride; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; Apo A: Apolipoprotein A; Apo B: Apolipoprotein B; LP(a): Lipoprotein a; FFAs: Free fatty acids
Ethics: The ethical approvals in order to study the effect of dietary oils on cardio-metabolic markers of patients with T2DM and bio-banking of blood fractions for both patients and their spouses were obtained from the ethics committee of Shahid Sadoughi University of Medical Sciences on 29th and 15th May 2016 with reference numbers IR.SSU.REC.1395.25 and IR.SSU.REC.1395.26, respectively. Furthermore, for studying the effect of dietary oils on cardio-metabolic markers in the patients’ spouses, who did not have diabetes, another ethics approval was obtained on 29th May 2016 with reference code IR.SSU.REC.1395.247 from the mentioned ethics committee.
The trial was registered in the Iranian Registry of Clinical Trials (IRCT) on 14th of November 2016 (registration ID: IRCT2016091312571N6), and archived at <https://en.irct.ir/trial/12622>. Informed consents were obtained from all study participants.
Inclusion criteria: Participants who were 18-60 years old, had a minimum of 6 months or a maximum of 10 years history of T2DM, took oral anti-glycemic agents as medication and did not take insulin therapy, had not changed the dose of lipid-lowering medications at least for 3 months prior to starting the study, and provided informed consent to entering the study were included in the present study. Furthermore, the participants should have HbA1c values of less than 8%, and no history of any other diseases like CVD (coronary artery disease, stroke, congestive heart disease) and coronary artery bypass grafting (CABG), kidney or liver diseases [serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic-pyruvic transaminase (SGPT) levels of three times higher than normal values], thyroid disease, and any types of cancer.
Exclusion criteria: Participants who dramatically changed their dietary habits during the study period or went on a special diet, underwent insulin therapy throughout the study period, experienced pregnancy or chronic diseases like CVD or cancer, or intended to discontinue the study for any reason were excluded from the study. We did not consider any inclusion or exclusion criteria for the spouses.
Sample size calculation: The sample size for the present study was calculated based on a formula suggested for crossover studies29 [n = [(z 1−α/2+z 1−β)2 · s 2]/2Δ2] which assumes the type one error of 5% and the type 2 error of 10% (power of 90%), and serum glucose as the key variable.14 Using this formula, a minimum of 34 participants was calculated as the required sample size. In the present study, we aimed to have enough power to conduct sex specific analyses. The investigators predicted that the attrition rate might be high in the present study; therefore, we targeted to enter 50 men and 50 women with the eligibility criteria.
Randomization: The participants were stratified based on their sex, and then, were randomly assigned to one of the 6 sequences of rolling methods in order to consume the three intervention oils during the study period (SGB, SBG, GSB, GBS, SGB or BSG) (Figure 1). The randomization was implemented using the Statistical Package for Social Sciences software (SPSS) (version 20, IBM Corporation, Armonk, NY, USA) by an independent researcher.
Allocation concealment: The pre-specified rolling methods were written on a paper and were kept in sealed opaque envelopes. At the initial visit, the envelope was opened by the study coordinator when the subject gave informed consent to be entered into the study.
Blinding: This study was designed to be a triple-blind trial. The intervention oils were provided in exactly the same bottles labeled with three codes (B, G, and S) by a responsible person who was not aware of the study objectives. The codes were not released until after the statistical analyses; therefore, neither the study participants (patients with diabetes and their spouses), nor the personnel and the statisticians were aware of the intervention oils until after the statistical analyses.
Chemical analysis of intervention oils: The fatty acids content of the intervention oils and the sunflower oil were assessed using gas chromatography with a flame ionizer detector (GC-FID) (model YL6500 GC, Young ling Instruments, Korea) before their delivery. Mean percentage of the important fatty acids content is provided in table 2. In brief, the fatty acids content of the intervention oils was as follows: 1) CO: 60.95% oleic acid, 8.048% ALA, and 21.87% linoleic acid, 2) SO: 40.95% oleic acid, 0.357% ALA, and 42.62% linoleic acid, and 3) SCO: 52.94% oleic acid, 4.98% ALA, and 30.17% linoleic acid (Table 2).
Table 2.
The fatty acid composition of treatment oils*
| Fatty acids | Canola oil | Sesame oil | Sesame-canola oil | Sunflower oil |
|---|---|---|---|---|
| Saturated fatty aids | ||||
| Palmitic acid (16:0) | 5.369 | 9.576 | 7.046 | 6.870 |
| Stearic acid (18:0) | 2.221 | 5.776 | 3.940 | 5.540 |
| Arachidic acid (20:0) | 0.295 | 0.379 | 0.330 | 0.360 |
| Behenic acid (22:0) | 0.265 | - | 0.156 | 0.540 |
| Lignoceric acid (24:0) | - | - | - | 0.190 |
| Monounsaturated fatty acids | ||||
| Palmitoleic acid (16:1) | 0.271 | 0.198 | 0.239 | 0.188 |
| Oleic acid (18:1) | 60.950 | 40.950 | 52.940 | 28.460 |
| Erucic acid (22:1) | 0.389 | - | 0.190 | |
| Polyunsaturated fatty acids | ||||
| Linoleic acid (18:2) | 21.870 | 42.620 | 30.170 | 57.450 |
| Alpha-linolenic acid (18:3) | 8.048 | 0.357 | 4.980 | 0.140 |
All values are presented as the percentages of total fatty acids.
Primary and secondary outcomes: The present clinical trial was designed to examine the effects of CO, SO, and SCO on fasting blood sugar (FBS) and serum lipid profile concentrations of TG, TC, high-density lipoprotein cholesterol (HDL-C), and LDL-C as the primary outcomes. The secondary outcomes were Apo A, Apo B, lipoprotein(a) [Lp (a)] concentration, blood pressure, and variation in anthropometric and body composition indices.
Anthropometrics measurements: Height was measured using a wall-fixed measuring tape to the nearest 0.1 cm. Moreover, waist and hip circumferences were measured to the nearest 1 cm using a non-stretchable measuring tape. Body weight was measured with light clothes and without shoes to the nearest 100 gr using a digital calibrated scale (mode BF51, Omron, Japan). Body mass index (BMI) is computed by dividing weight (kg) by height squared (m2), and the waist to hip ratio (WHR) by dividing waist circumference by hip circumference. Visceral fat, lean mass, and body fat percentage were also assessed using a body composition analyzer (model BF51, Omron, Japan). All anthropometric assessments were performed 3 times in each visit and their mean value was recorded as the final value (Table 1).
Blood pressure measurement: Systolic and diastolic blood pressure (SBP and DBP) were measured 3 times in each visit after 5 minutes rest when participants were in the sitting mode, for the right arm with at least 1-minute interval, using a sphygmomanometer (Riester, Germany, model: Diplomat-presameter). The mean of SBP and DBP values was recorded for each visit (Table 1).
Blood sampling: After an overnight fast (10-12 hours), venous blood samples were taken from participants and their spouses between 7-9:30 a.m. in the morning. The blood samples were aliquoted to 3 serums, 3 plasmas, 2 buffy coat, and 2 whole blood samples in DNase- and RNase-free microtubes and stored at -80 ºC until analysis.
Laboratory assessment: FBS, TG, TC, HDL-C, LDL-C, Apo A, Apo B, and Lp (a) were determined from serum samples using an auto-analyzer (model AT++, Alpha-classic, Iran) and Pars Azmoon standard kits (Pars Azmoon Inc., Iran) (Table 1).
Dietary intake measurement: In this trial, 3-day weighted food record (2 weekdays and 1 day of the weekend) was used to measure dietary nutrients intake, including energy, carbohydrate, protein, total fat, saturated fat, MUFAs, and PUFAs intake at the beginning, in the middle, and at the end of the intervention. Therefore, the food records were collected 9 times during the study (Table 1). Participants were instructed by a nutritionist to fill out the food records in the initial visit and were provided with written instructions. The food records were completed by all of the participants. They were asked to record the type and amount of all foods, beverages, supplements, and medications consumed. A digital kitchen scale (model SF-400, Electronic kitchen scale, China) was provided for each participant or the person who was responsible for cooking at home and they were asked to complete the 3-day cooking forms for each visit. The weight of every cooked food and its ingredients were also recorded. The daily food intakes will be computed and converted to grams/day using household measures.30 Daily energy and nutrients intakes will be calculated using a version of the Nutritionist IV software (version 3.5.2, Axxya Systems, Redmond, Washington, DC, USA) modified for Iranian foods.
Physical activity assessment: Physical activity was assessed using 3-day physical activity records (2 weekdays and 1 day of the weekend). The records were collected 9 times during the study (at the beginning, in the middle, and at the end of each phase). The physical activity data will be converted into metabolic equivalent-min/day (Table 1) using the updated version of the compendium of physical activities.31 The participants were asked to keep their physical activity level constant during the study.
Compliance: The intervention oils were provided for the participants and their family. Thus, to evaluate compliance, several methods were implemented; 1) the given and returned intervention oil bottles were weighed and will be divided by the number of members usually living in the participants’ house, and 2) the 3-day food records will be used to assess the amount of oil consumed by the participants (Table 1).
Medication use: To track the medications used by the participants, they were asked to record the medications and their dose in the food records and the medication use was evaluated at each clinical assessment visit; therefore, the possible changes in medications will be accessible to the study participants (Table 1).
Data management: All data will be kept in the office of the principal investigator (ASA) and will be available only to the investigators for research purposes. The collected data will be entered into a data file and will be kept secure by the principal investigators. The data will also include patients' medical history information. The access to the data will be limited to statistical analyses and interpretation. The collected data will not be used for any other purposes. The biological samples will be kept in a freezer until the analyses and only the principal investigator will have access to the samples. The biological samples will be used only for research purposes.
Confidentiality of the data: All collected data for the present investigation will remain confidential, and the investigators will follow the ethical standards of Shahid Sadoughi University of Medical Sciences. However, the clinical research members of the research team will be aware of the identity of the participants, but not of the intervention oils provided for the study participants during the study.
Each participant will receive a unique identification code so that all information, such as data gathered using questionnaires, measurements, and biological samples, will remain confidential. Full names and other identifying information will not be provided, unless required by law and/or by the research ethics board. The participants will not be identified in any published data or in any result from this study. Moreover, medical records that contain the identity of the participants will be regarded as confidential.
Participant feedback: The report of the primary outcomes will be provided for the study participants in sealed envelopes and in a private meeting with the investigators, as soon as the analyses become completed.
Adverse events and concomitant medications: No adverse reactions were reported during the study period and we did not expect any adverse events because the intervention components were food and they were readily available for people in food stores. The case report form was designed to record any adverse events and to inform sponsors and the institutional ethics board of Shahid Sadoughi University of Medical Sciences. All medications used at the beginning of the trial or during the study period were recorded.
The auditing of the present study was performed by two independent investigators who were not a study team member or sponsor during the recruitment and follow-up periods.
Study status: The study is still ongoing. The recruitment of participants started in April 2016 and the intervention period ended in May 2017. The biochemical assessments of the blood samples are currently being carried out. Furthermore, the investigators are now entering the data and preparing them for statistical analysis in the near future. The project has not led to any publication yet.
Statistical analysis: The statistical analysis will be conducted using IBM SPSS statistical software. The normality of the distribution of the quantitative data will be determined using Kolmogorov-Smirnov test, and the skewed variables will be normalized by transformation before comparison. Baseline and post-intervention measurements will be compared using repeated measures analysis of variance (ANOVA) to determine treatment effects. The effects of treatment oils will be compared using linear mixed method procedure with rolling method between subject factors. The potential confounders like age, sex, baseline BMI, the amount of intervention oils consumed per subject, metabolic equivalent-min/day of physical activity, and the amount of calorie intake will be adjusted as covariates. Sex specific analyses will be conducted. Sensitivity analysis will be performed by excluding those who experienced medication change throughout the study. P < 0.050 will be considered as statistically significant for all analyses. The results related to the intervention oils will be compared using the Bonferroni adjustment for multiple comparisons.
Results
Enrollment and dropouts: Among 574 individuals with diabetes, 162 participants underwent the clinical assessments and 114 individuals met the inclusion criteria. In addition, 12 eligible participants did not intend to enter the project through the run-in period. Eventually, 102 participants were randomized to one of the 6 rolling methods (Figure 1).
In phase 1, 2, and 3, respectively, 5, 2, and 2 participants did not participate in the visits; therefore, 93 (91.2%) participants completed all of the study phases and the overall dropout rate was 8.8%. These participants did not continue the study because of the following reasons: did not intend to continue the participation (n = 3), did not intend to give blood sample in at least one visit with blood sampling (n = 3), moved to another city (n = 1), went on insulin therapy (n = 1), and experienced CVD during the study (n = 1).
Discussion
Canola oil (CO) is a good source of oleic acid, ALA, and phytochemicals.12 Canola has risen from the sixth largest oil crop to the second in the last 40 years and CO is the third largest source of edible plant oil in the world.32 Several studies have reported the significant improvements in blood lipids,33-36 FBS,15,16 blood pressure,37 and insulin sensitivity16,17 as a result of consuming a CO-based diet. In a crossover clinical trial with a 3-week intervention period on 20 participants, a CO-enriched diet significantly decreased FBS and lipid profile.15 Additionally, a 12-week parallel intervention on 70 patients with T2DM indicated a significant reduction in FBS, weight, lipid profile, and blood pressure after CO intake.14 Furthermore, SO is recognized as a source of high amounts of lignans (sesamin, sesamolin, and sesaminol) and vitamin E, and due to its acting as an antioxidant.19,21,22 In addition, for over 4000 years, sesame has been grown worldwide particularly in tropical and semi-tropical climates, sandy soils, and under droughty conditions.38 The health benefits of SO have attracted the attention of many researchers from Asian countries because of its high consumption rate in this area.24,39 In a parallel trial performed by Sankar et al. on 356 patients with hypertension, SO elevated HDL-C and reduced lipid peroxidation in 6 weeks.24 This study also reported the beneficial effects of SO on lipid profile, and enzymatic and non-enzymatic antioxidants.24 A meta-analysis also found that SO consumption significantly reduced TG.40 SO may improve both SBP and DBP,19,24 and decrease the lipogenic enzyme activities.41 Additionally, the anti-atherosclerotic properties of SO were shown in an animal study.23 Although both CO and SO are considered as healthy dietary vegetable oils, the effects of these two vegetable oils have not yet been compared. The present study was performed to compare the effect of replacing regular oil consumed by participants with T2DM and their spouses with SO, CO, and SCO on cardio-metabolic markers.
Strengths and limitations: This study was a three-way crossover study in which participants acted as their own controls. Compared to parallel-arm designs, crossover studies are proposed to have more precision and statistical power,42 and minimize the confounding variables.43 A recent systematic review reported that few crossover clinical trials have assessed the effect of SO on lipid profile in humans.40 Furthermore, the mentioned systematic review reported that the intervention duration in the majority of crossover studies regarding the effect of SO on cardio-metabolic markers was less than 6 weeks, which is true for studies assessing the effects of CO as well.15,18,19,34,37,38,44-55
Additionally, with 93 study completers and enough biological samples, the present study enables researchers to examine the sex stratified effect of the intervention oils and different markers with high statistical power. Furthermore, this sample size will make studying gene-diet interactions possible in the future. Some strategies such as monthly visits, providing personalized results, and phone follow-ups motivated individuals to continue the study; thus, this trial was completed with a low attrition rate (8.8%). In the present study, we tried to improve the quality using standard methods for randomization, allocation concealment, and blinding which minimize the potential selection bias, confounding factors, and ascertainment bias.56,57
Furthermore, the present study, with a 9-week duration for each intervention phase, has a long period of intervention among crossover studies that have assessed the effect of CO15,33,36,37,45,47-54,58,59 and SO19,43 on different cardio-metabolic markers. The current study examined SCO, the blend of sesame and canola oil, as a new oil product and we are not aware of any similar studies. Additionally, in this trial, we aimed to replace the common oil intake of participants to assess the effects of the mentioned oils on their routine life.
Medications used by participants were recorded on each visiting day, and the investigators will be able to check the sensitivity of the results by removing those whose medications were changed. All the procedures reported in the present study were also performed for the participants’ spouses, and thus, it is possible to investigate the effect of the intervention oils in adults without diabetes.
It should be noted that the exact amount of oil consumed by each person will not be clear; however, we tried to resolve this problem by asking the study participants and their spouses to report the mount of oil consumed as tablespoon in their food records, and the weight of oil provided before and after consumption in each phase was assessed.
Future investigations: The investigators are planning to compare the effect of the intervention oils on markers of glucose control including fasting serum insulin, homeostatic model assessment of insulin resistance (HOMA_IR) and quantitative insulin sensitivity check index (QUICKI), blood markers of kidney function (blood urea nitrogen and serum creatinine), liver enzymes [SGOT, SGPT, alkaline phosphatase (Alp), and gamma-glutamyl transferase (GGT)], markers of oxidative stress, and inflammatory markers in participants with T2DM and their spouses in the near future. The samples will also allow the investigators to examine the possible interactions between gene polymorphisms and intervention oils on cardio-metabolic markers. The principal investigators welcome possible collaborations with interested scientists and novel hypotheses that could be checked using the available samples and data obtained in the current study.
Conclusion
In summary, the current three-way, triple-blind, clinical trial will investigate the effect of replacing regular oil consumed by participants with T2DM and their spouses with SO, CO, and SCO (the blend of sesame and canola oil; a new oil production) on cardio-metabolic markers, anthropometric indices, and blood pressure. This study, with its large sample size and bio-banking of different fractions of blood, will provide the opportunity to explore the effect of dietary oils on different aspects of human health.
Acknowledgments
We would like to thank the participants for their voluntary and enthusiastic involvement in the project. Moreover, we would like to thank Shahid Sadoughi University of Medical Sciences and Neshatavar food industry company (Datis Corporation) for their joint funding of this study. The authors would also like to thank the research council of Nutrition and Food Security Research Center for their scientific support, and the Diabetes Research Center of Shahid Sadoughi University of Medical Sciences, Yazd, Iran, for their close cooperation and their executive assistance.
Trial registration: The trial was registered at the Iranian Registry of Clinical trials (http:/www.irct.ir) with the registration code IRCT2016091312571N6 in November 2016.
Footnotes
Conflicts of Interest
The study was jointly funded by Shahid Sadoughi University of Medical sciences and Datis Corporation. Datis Corporation did not take any part in the conception, design, and execution of the study protocol, and the reporting of the study results. The corporation did not have any other relationship with the investigators. The authors declare that they have no other potential personal or financial conflicts of interest. The principal investigator (ASA) declares that he has full access to the data and samples provided by this project.
REFERENCES
- 1.Panagiotakos DB, Georgousopoulou EN, Pitsavos C, Chrysohoou C, Skoumas I, Pitaraki E, et al. Exploring the path of Mediterranean diet on 10-year incidence of cardiovascular disease: The ATTICA study (2002-2012). Nutr Metab Cardiovasc Dis. 2015;25(3):327–35. doi: 10.1016/j.numecd.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris WS. n-3 fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr. 1997;65(5 Suppl):1645S–54S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 4.Sanders TA, Oakley FR, Miller GJ, Mitropoulos KA, Crook D, Oliver MF. Influence of n-6 versus n-3 polyunsaturated fatty acids in diets low in saturated fatty acids on plasma lipoproteins and hemostatic factors. Arterioscler Thromb Vasc Biol. 1997;17(12):3449–60. doi: 10.1161/01.atv.17.12.3449. [DOI] [PubMed] [Google Scholar]
- 5.Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: An 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29(7):1354–67. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Forouhi NG, Imamura F, Sharp SJ, Koulman A, Schulze MB, Zheng J, et al. Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: The EPIC-InterAct case-cohort study. PLoS Med. 2016;13(7):e1002094. doi: 10.1371/journal.pmed.1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heine RJ, Mulder C, Popp-Snijders C, van der Meer J, van der Veen EA. Linoleic-acid-enriched diet: Long-term effects on serum lipoprotein and apolipoprotein concentrations and insulin sensitivity in noninsulin-dependent diabetic patients. Am J Clin Nutr. 1989;49(3):448–56. doi: 10.1093/ajcn/49.3.448. [DOI] [PubMed] [Google Scholar]
- 8.Lombardo YB, Chicco AG. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J Nutr Biochem. 2006;17(1):1–13. doi: 10.1016/j.jnutbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Roche HM, Gibney MJ. Long-chain n-3 polyunsaturated fatty acids and triacylglycerol metabolism in the postprandial state. Lipids. 1999;34(Suppl):S259–S265. doi: 10.1007/BF02562313. [DOI] [PubMed] [Google Scholar]
- 10.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 11.Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, et al. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond) 2006;30(10):1535–44. doi: 10.1038/sj.ijo.0803309. [DOI] [PubMed] [Google Scholar]
- 12.Dittrich M, Jahreis G, Bothor K, Drechsel C, Kiehntopf M, Bluher M, et al. Benefits of foods supplemented with vegetable oils rich in alpha-linolenic, stearidonic or docosahexaenoic acid in hypertriglyceridemic subjects: A double-blind, randomized, controlled trail. Eur J Nutr. 2015;54(6):881–93. doi: 10.1007/s00394-014-0764-2. [DOI] [PubMed] [Google Scholar]
- 13.Baxheinrich A, Stratmann B, Lee-Barkey YH, Tschoepe D, Wahrburg U. Effects of a rapeseed oil-enriched hypoenergetic diet with a high content of alpha-linolenic acid on body weight and cardiovascular risk profile in patients with the metabolic syndrome. Br J Nutr. 2012;108(4):682–91. doi: 10.1017/S0007114512002875. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins DJ, Kendall CW, Vuksan V, Faulkner D, Augustin LS, Mitchell S, et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: A randomized controlled trial. Diabetes Care. 2014;37(7):1806–14. doi: 10.2337/dc13-2990. [DOI] [PubMed] [Google Scholar]
- 15.Iggman D, Gustafsson IB, Berglund L, Vessby B, Marckmann P, Riserus U. Replacing dairy fat with rapeseed oil causes rapid improvement of hyperlipidaemia: A randomized controlled study. J Intern Med. 2011;270(4):356–64. doi: 10.1111/j.1365-2796.2011.02383.x. [DOI] [PubMed] [Google Scholar]
- 16.Nigam P, Bhatt S, Misra A, Chadha DS, Vaidya M, Dasgupta J, et al. Effect of a 6-month intervention with cooking oils containing a high concentration of monounsaturated fatty acids (olive and canola oils) compared with control oil in male Asian Indians with nonalcoholic fatty liver disease. Diabetes Technol Ther. 2014;16(4):255–61. doi: 10.1089/dia.2013.0178. [DOI] [PubMed] [Google Scholar]
- 17.Kruse M, von Loeffelholz C, Hoffmann D, Pohlmann A, Seltmann AC, Osterhoff M, et al. Dietary rapeseed/canola-oil supplementation reduces serum lipids and liver enzymes and alters postprandial inflammatory responses in adipose tissue compared to olive-oil supplementation in obese men. Mol Nutr Food Res. 2015;59(3):507–19. doi: 10.1002/mnfr.201400446. [DOI] [PubMed] [Google Scholar]
- 18.Sodergren E, Gustafsson IB, Basu S, Nourooz-Zadeh J, Nalsen C, Turpeinen A, et al. A diet containing rapeseed oil-based fats does not increase lipid peroxidation in humans when compared to a diet rich in saturated fatty acids. Eur J Clin Nutr. 2001;55(11):922–31. doi: 10.1038/sj.ejcn.1601246. [DOI] [PubMed] [Google Scholar]
- 19.Sankar D, Rao MR, Sambandam G, Pugalendi KV. A pilot study of open label sesame oil in hypertensive diabetics. J Med Food. 2006;9(3):408–12. doi: 10.1089/jmf.2006.9.408. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho RH, Galvao EL, Barros JA, Conceicao MM, Sousa EM. Extraction, fatty acid profile and antioxidant activity of sesame extract (Sesamum Indicum L.). Braz J Chem Eng. 2012;29(2):409–20. [Google Scholar]
- 21.Matsumura Y. The anti-hypertensive effect of sesamin. In: Tan BK, Bay BH, Zhu YZ, editors. Novel Compounds from natural products in the new millennium: Potential and challenges. Singapore, Singapore: World Scientific; 1998. p. 170. [Google Scholar]
- 22.Kita S, Matsumura Y, Morimoto S, Akimoto K, Furuya M, Oka N, et al. Antihypertensive effect of sesamin. II. Protection against two-kidney, one-clip renal hypertension and cardiovascular hypertrophy. Biol Pharm Bull. 1995;18(9):1283–5. doi: 10.1248/bpb.18.1283. [DOI] [PubMed] [Google Scholar]
- 23.Bhaskaran S, Santanam N, Penumetcha M, Parthasarathy S. Inhibition of atherosclerosis in low-density lipoprotein receptor-negative mice by sesame oil. J Med Food. 2006;9(4):487–90. doi: 10.1089/jmf.2006.9.487. [DOI] [PubMed] [Google Scholar]
- 24.Sankar D, Sambandam G, Ramakrishna RM, Pugalendi KV. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta. 2005;355(1-2):97–104. doi: 10.1016/j.cccn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Mitra A. Study on the benefits of sesame oil over coconut oil in patients of insulin resistance syndrome, notably type 2 diabetes and dyslipidaemia. J Hum Ecol. 2007;22(1):61–6. [Google Scholar]
- 26.Goff DC, Gerstein HC, Ginsberg HN, Cushman WC, Margolis KL, Byington RP, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: Current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99(12A):4i–20i. doi: 10.1016/j.amjcard.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 29.Chow SC, Wang H, Shao J. Sample size calculations in clinical research. 2nd. Boca Raton, FL: CRC Press; 2007. [Google Scholar]
- 30.Houshiar-Rad A, Ghaffarpour M, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran, Iran: Keshaverzi Publications; 1999. In Persian. [Google Scholar]
- 31.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 32.Takeyama N, Shoji Y, Ohashi K, Tanaka T. Role of reactive oxygen intermediates in lipopolysaccharide-mediated hepatic injury in the rat. J Surg Res. 1996;60(1):258–62. doi: 10.1006/jsre.1996.0040. [DOI] [PubMed] [Google Scholar]
- 33.Valsta LM, Jauhiainen M, Aro A, Katan MB, Mutanen M. Effects of a monounsaturated rapeseed oil and a polyunsaturated sunflower oil diet on lipoprotein levels in humans. Arterioscler Thromb. 1992;12(1):50–7. doi: 10.1161/01.atv.12.1.50. [DOI] [PubMed] [Google Scholar]
- 34.Wardlaw GM, Snook JT, Lin MC, Puangco MA, Kwon JS. Serum lipid and apolipoprotein concentrations in healthy men on diets enriched in either canola oil or safflower oil. Am J Clin Nutr. 1991;54(1):104–10. doi: 10.1093/ajcn/54.1.104. [DOI] [PubMed] [Google Scholar]
- 35.Gustafsson IB, Vessby B, Ohrvall M, Nydahl M. A diet rich in monounsaturated rapeseed oil reduces the lipoprotein cholesterol concentration and increases the relative content of n-3 fatty acids in serum in hyperlipidemic subjects. Am J Clin Nutr. 1994;59(3):667–74. doi: 10.1093/ajcn/59.3.667. [DOI] [PubMed] [Google Scholar]
- 36.Nydahl M, Gustafsson IB, Ohrvall M, Vessby B. Similar effects of rapeseed oil (canola oil) and olive oil in a lipid-lowering diet for patients with hyperlipoproteinemia. J Am Coll Nutr. 1995;14(6):643–51. doi: 10.1080/07315724.1995.10718554. [DOI] [PubMed] [Google Scholar]
- 37.Chisholm A, Mc Auley, Mann J, Williams S, Skeaff M. Cholesterol lowering effects of nuts compared with a Canola oil enriched cereal of similar fat composition. Nutr Metab Cardiovasc Dis. 2005;15(4):284–92. doi: 10.1016/j.numecd.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Hsu DZ, Liu MY. Sesame oil protects against lipopolysaccharide-stimulated oxidative stress in rats. Crit Care Med. 2004;32(1):227–31. doi: 10.1097/01.CCM.0000104947.16669.29. [DOI] [PubMed] [Google Scholar]
- 39.Sankar D, Ali A, Sambandam G, Rao R. Sesame oil exhibits synergistic effect with anti-diabetic medication in patients with type 2 diabetes mellitus. Clin Nutr. 2011;30(3):351–8. doi: 10.1016/j.clnu.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Khalesi S, Paukste E, Nikbakht E, Khosravi-Boroujeni H. Sesame fractions and lipid profiles: A systematic review and meta-analysis of controlled trials. Br J Nutr. 2016;115(5):764–73. doi: 10.1017/S0007114515005012. [DOI] [PubMed] [Google Scholar]
- 41.Hirose N, Inoue T, Nishihara K, Sugano M, Akimoto K, Shimizu S, et al. Inhibition of cholesterol absorption and synthesis in rats by sesamin. J Lipid Res. 1991;32(4):629–38. [PubMed] [Google Scholar]
- 42.Mackay DS, Jew S, Jones PJ. Best practices for design and implementation of human clinical trials studying dietary oils. Prog Lipid Res. 2017;65:1–11. doi: 10.1016/j.plipres.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, Bakhai A. Clinical Trials: A practical guide to design, analysis, and reporting. Limassol, Cyprus: Remedica; 2006. [Google Scholar]
- 44.Sankar D, Rao MR, Sambandam G, Pugalendi KV. Effect of sesame oil on diuretics or Beta-blockers in the modulation of blood pressure, anthropometry, lipid profile, and redox status. Yale J Biol Med. 2006;79(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- 45.Jones PJ, Senanayake VK, Pu S, Jenkins DJ, Connelly PW, Lamarche B, et al. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am J Clin Nutr. 2014;100(1):88–97. doi: 10.3945/ajcn.113.081133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen LF, Jespersen J, Marckmann P. Are olive oil diets antithrombotic? Diets enriched with olive, rapeseed, or sunflower oil affect postprandial factor VII differently. Am J Clin Nutr. 1999;70(6):976–82. doi: 10.1093/ajcn/70.6.976. [DOI] [PubMed] [Google Scholar]
- 47.Lichtenstein AH, Ausman LM, Carrasco W, Gualtieri LJ, Jenner JL, Ordovas JM, et al. Rice bran oil consumption and plasma lipid levels in moderately hypercholesterolemic humans. Arterioscler Thromb. 1994;14(4):549–56. doi: 10.1161/01.atv.14.4.549. [DOI] [PubMed] [Google Scholar]
- 48.McDonald BE, Gerrard JM, Bruce VM, Corner EJ. Comparison of the effect of canola oil and sunflower oil on plasma lipids and lipoproteins and on in vivo thromboxane A2 and prostacyclin production in healthy young men. Am J Clin Nutr. 1989;50(6):1382–8. doi: 10.1093/ajcn/50.6.1382. [DOI] [PubMed] [Google Scholar]
- 49.McKenney JM, Proctor JD, Wright JT, Kolinski RJ, Elswick RK, Coaker JS. The effect of supplemental dietary fat on plasma cholesterol levels in lovastatin-treated hypercholesterolemic patients. Pharmacotherapy. 1995;15(5):565–72. doi: 10.1002/j.1875-9114.1995.tb02864.x. [DOI] [PubMed] [Google Scholar]
- 50.Ohrvall M, Gustafsson IB, Vessby B. The alpha and gamma tocopherol levels in serum are influenced by the dietary fat quality. J Hum Nutr Diet. 2001;14(1):63–8. doi: 10.1046/j.1365-277x.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 51.Truswell AS, Choudhury N, Roberts DCK. Double blind comparison of plasma lipids in healthy subjects eating potato crisps fried in palmolein or canola oil. Nutr Res. 1992;12:S43–S52. [Google Scholar]
- 52.Uusitupa M, Schwab U, Makimattila S, Karhapaa P, Sarkkinen E, Maliranta H, et al. Effects of two high-fat diets with different fatty acid compositions on glucose and lipid metabolism in healthy young women. Am J Clin Nutr. 1994;59(6):1310–6. doi: 10.1093/ajcn/59.6.1310. [DOI] [PubMed] [Google Scholar]
- 53.Karvonen HM, Tapola NS, Uusitupa MI, Sarkkinen ES. The effect of vegetable oil-based cheese on serum total and lipoprotein lipids. Eur J Clin Nutr. 2002;56(11):1094–101. doi: 10.1038/sj.ejcn.1601452. [DOI] [PubMed] [Google Scholar]
- 54.Fialho Lopes DC, Coelho Silvestre MP, Medeiros Silva VD, Moreira TC, Garcia ES, Silva MR. Dietary Supplementation of Conjugated Linoleic Acid, Added to a Milk Drink, in Women. Asian J Sci Res. 2013;6:679–90. [Google Scholar]
- 55.Sundram K, Hayes KC, Siru OH. Both dietary 18:2 and 16:0 may be required to improve the serum LDL/HDL cholesterol ratio in normocholesterolemic men. J Nutr Biochem. 1995;6(4):179–87. [Google Scholar]
- 56.Berger VW. A review of methods for ensuring the comparability of comparison groups in randomized clinical trials. Rev Recent Clin Trials. 2006;1(1):81–6. doi: 10.2174/157488706775246139. [DOI] [PubMed] [Google Scholar]
- 57.Senior H. Randomization, allocation concealment, and blinding. In: Nikles J, Mitchell G, editors. The essential guide to N-of-1 trials in health. Berlin, Germany: Springer; 2015. pp. 81–91. [Google Scholar]
- 58.Albert BB, Derraik JG, Brennan CM, Biggs JB, Garg ML, Cameron-Smith D, et al. Supplementation with a blend of krill and salmon oil is associated with increased metabolic risk in overweight men. Am J Clin Nutr. 2015;102(1):49–57. doi: 10.3945/ajcn.114.103028. [DOI] [PubMed] [Google Scholar]
- 59.Lichtenstein AH, Ausman LM, Carrasco W, Jenner JL, Gualtieri LJ, Goldin BR, et al. Effects of canola, corn, and olive oils on fasting and postprandial plasma lipoproteins in humans as part of a National Cholesterol Education Program Step 2 diet. Arterioscler Thromb. 1993;13(10):1533–42. doi: 10.1161/01.atv.13.10.1533. [DOI] [PubMed] [Google Scholar]