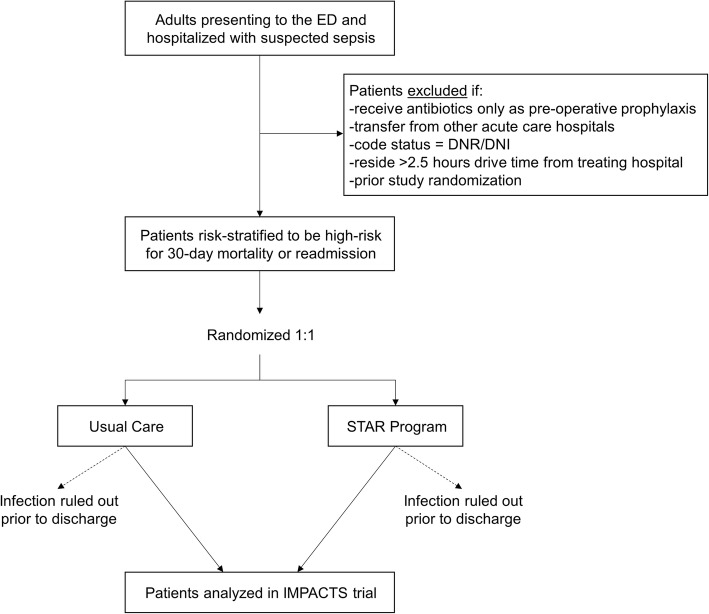
Fig. 1.
Patient flow diagram for participation in THE IMPACTS trial. The study population includes adults presenting to the Emergency Department (ED) who meet the following inclusion criteria: ≥ 18 years of age; oral or parenteral antibiotic or bacterial culture order within 24 h of ED presentation and either culture drawn first, antibiotics ordered within 48 h or antibiotics ordered first, culture ordered within 48 h; not discharged from the hospital at the time the daily list of eligible patients is generated each weekday morning; and deemed high risk for either 30-day readmission or mortality using risk-scoring models applied daily to real-time clinical data on acute and chronic factors. Patients are excluded based on receipt of prophylactic antibiotics only, hospital transfers, “do not resuscitate” or “do not intubate” (DNR/DNI) code status, distance of residence from treating hospital, and prior study randomization. Patients who have infection ruled out prior to hospital discharge are also excluded. IMPACTS Improving Morbidity during Post-Acute Care Transitions for Sepsis, STAR Sepsis Transition and Recovery